Structural biology of human GPCR drugs and endogenous ligands - insights from NMR spectroscopy
- PMID: 32911074
- PMCID: PMC7572649
- DOI: 10.1016/j.ymeth.2020.08.008
Structural biology of human GPCR drugs and endogenous ligands - insights from NMR spectroscopy
Abstract
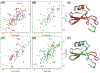
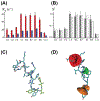
G protein-coupled receptors (GPCRs) represent the largest class of "druggable" proteins in the human genome. For more than a decade, crystal structures and, more recently, cryoEM structures of GPCR complexes have provided unprecedented insight into GPCR drug binding and cell signaling. Nevertheless, structure determination of receptors in complexes with weakly binding molecules or complex polypeptides remains especially challenging, including for hormones, many of which have so far eluded researchers. Nuclear magnetic resonance (NMR) spectroscopy has emerged as a promising approach to determine structures of ligands bound to their receptors and to provide insights into the dynamics of GPCR-bound drugs. The capability to investigate compounds with weak binding affinities has also been leveraged in NMR applications to identify novel lead compounds in drug screening campaigns. We review recent structural biology studies of GPCR ligands by NMR, highlighting new methodologies enabling studies of GPCRs with native sequences and in native-like membrane environments that provide insights into important drugs and endogenous ligands.
Keywords: Endogenous ligands; GPCR drugs; Integrative structural biology; Membrane proteins; NMR spectroscopy.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



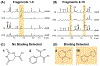
Similar articles
-
GPCR drug discovery: integrating solution NMR data with crystal and cryo-EM structures.Nat Rev Drug Discov. 2019 Jan;18(1):59-82. doi: 10.1038/nrd.2018.180. Epub 2018 Nov 9. Nat Rev Drug Discov. 2019. PMID: 30410121 Free PMC article. Review.
-
Solution- and solid-state NMR studies of GPCRs and their ligands.Biochim Biophys Acta. 2011 Jun;1808(6):1462-75. doi: 10.1016/j.bbamem.2010.10.003. Epub 2010 Oct 15. Biochim Biophys Acta. 2011. PMID: 20951674 Review.
-
Unraveling the structure and function of G protein-coupled receptors through NMR spectroscopy.Curr Pharm Des. 2009;15(35):4003-16. doi: 10.2174/138161209789824803. Curr Pharm Des. 2009. PMID: 20028318 Free PMC article. Review.
-
Dynamic G Protein-Coupled Receptor Signaling Probed by Solution NMR Spectroscopy.Biochemistry. 2020 Mar 17;59(10):1065-1080. doi: 10.1021/acs.biochem.0c00032. Epub 2020 Mar 2. Biochemistry. 2020. PMID: 32092261
-
G-protein-coupled receptor structure, ligand binding and activation as studied by solid-state NMR spectroscopy.Biochem J. 2013 Mar 15;450(3):443-57. doi: 10.1042/BJ20121644. Biochem J. 2013. PMID: 23445222 Review.
Cited by
-
Function-Related Dynamics in Multi-Spanning Helical Membrane Proteins Revealed by Solution NMR.Membranes (Basel). 2021 Aug 9;11(8):604. doi: 10.3390/membranes11080604. Membranes (Basel). 2021. PMID: 34436367 Free PMC article. Review.
-
Global insights into the fine tuning of human A2AAR conformational dynamics in a ternary complex with an engineered G protein viewed by NMR.Cell Rep. 2022 Dec 20;41(12):111844. doi: 10.1016/j.celrep.2022.111844. Cell Rep. 2022. PMID: 36543140 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

