Direct Delivery of Apatite Nanoparticle-Encapsulated siRNA Targeting TIMP-1 for Intractable Abnormal Scars
- PMID: 32911344
- PMCID: PMC7486579
- DOI: 10.1016/j.omtn.2020.08.005
Direct Delivery of Apatite Nanoparticle-Encapsulated siRNA Targeting TIMP-1 for Intractable Abnormal Scars
Abstract
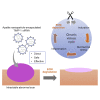
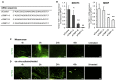
Hypertrophic scars (HSs) and keloids are histologically characterized by excessive extracellular matrix (ECM) deposition. ECM deposition depends on the balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteases (TIMPs). TIMP-1 has been linked to ECM degradation and is therefore a promising therapeutic strategy. In this study, we generated super carbonate apatite (sCA) nanoparticle-encapsulated TIMP-1 small interfering RNA (siRNA) (siTIMP1) preparations and examined the effect of local injections on mouse HSs and on ex vivo-cultured keloids. The sCA-siTIMP1 injections significantly reduced scar formation, scar cross-sectional areas, collagen densities, and collagen types I and III levels in the lesions. None of the mice died or exhibited abnormal endpoints. Apatite accumulation was not detected in the other organs. In an ex vivo keloid tissue culture system, sCA-siTIMP1 injections reduced the thickness and complexity of collagen bundles. Our results showed that topical sCA-siTIMP1 injections during mechanical stress-induced HS development reduced scar size. When keloids were injected three times with sCA-siTIMP1 during 6 days, keloidal collagen levels decreased substantially. Accordingly, sCA-siRNA delivery may be an effective approach for keloid treatment, and further investigations are needed to enable its practical use.
Keywords: ECM; TIMP; abnormal scar; hyprtrophic scar; keloid; nanoparticle; siRNA.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







Similar articles
-
Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids.J Plast Reconstr Aesthet Surg. 2010 Jun;63(6):1015-21. doi: 10.1016/j.bjps.2009.04.021. Epub 2009 May 22. J Plast Reconstr Aesthet Surg. 2010. PMID: 19464975
-
siRNA knockdown of tissue inhibitor of metalloproteinase-1 in keloid fibroblasts leads to degradation of collagen type I.J Invest Dermatol. 2014 Mar;134(3):818-826. doi: 10.1038/jid.2013.396. Epub 2013 Sep 16. J Invest Dermatol. 2014. PMID: 24042342
-
Hypertrophic and keloid scars fail to progress from the CD34- /α-smooth muscle actin (α-SMA)+ immature scar phenotype and show gradient differences in α-SMA and p16 expression.Br J Dermatol. 2020 Apr;182(4):974-986. doi: 10.1111/bjd.18219. Epub 2019 Sep 4. Br J Dermatol. 2020. PMID: 31206605
-
Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars.Exp Dermatol. 2021 Jan;30(1):146-161. doi: 10.1111/exd.14121. Epub 2020 Jul 6. Exp Dermatol. 2021. PMID: 32479693 Free PMC article. Review.
-
Photodynamic therapy for keloids and hypertrophic scars: a review.Scars Burn Heal. 2020 Jun 30;6:2059513120932059. doi: 10.1177/2059513120932059. eCollection 2020 Jan-Dec. Scars Burn Heal. 2020. PMID: 32655901 Free PMC article. Review.
Cited by
-
The EGF Motif With CXDXXXXYXCXC Sequence Suppresses Fibrosis in a Mouse Skin Wound Model.In Vivo. 2023 Jul-Aug;37(4):1486-1497. doi: 10.21873/invivo.13233. In Vivo. 2023. PMID: 37369508 Free PMC article.
-
Therapeutic Potential of Compounds with High Affinity to BAG2 in Inhibiting Keloid Disease.Biologics. 2025 Aug 24;19:497-510. doi: 10.2147/BTT.S533286. eCollection 2025. Biologics. 2025. PMID: 40893173 Free PMC article.
-
siRNA-Mediated Timp1 Silencing Inhibited the Inflammatory Phenotype during Acute Lung Injury.Int J Mol Sci. 2023 Jan 13;24(2):1641. doi: 10.3390/ijms24021641. Int J Mol Sci. 2023. PMID: 36675165 Free PMC article.
-
Use of Adipose Stem Cells Against Hypertrophic Scarring or Keloid.Front Cell Dev Biol. 2022 Jan 6;9:823694. doi: 10.3389/fcell.2021.823694. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35071247 Free PMC article. Review.
-
Non-coding RNAs are key players and promising therapeutic targets in atherosclerosis.Front Cell Dev Biol. 2023 Sep 1;11:1237941. doi: 10.3389/fcell.2023.1237941. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37719883 Free PMC article. Review.
References
-
- Jumper N., Paus R., Bayat A. Functional histopathology of keloid disease. Histol. Histopathol. 2015;30:1033–1057. - PubMed
-
- Lee J.Y., Yang C.C., Chao S.C., Wong T.W. Histopathological differential diagnosis of keloid and hypertrophic scar. Am. J. Dermatopathol. 2004;26:379–384. - PubMed
-
- Ogawa R., Okai K., Tokumura F., Mori K., Ohmori Y., Huang C., Hyakusoku H., Akaishi S. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012;20:149–157. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

