Medication adherence and rate of nicotine metabolism are associated with response to treatment with varenicline among smokers with HIV
- PMID: 32911350
- PMCID: PMC7572610
- DOI: 10.1016/j.addbeh.2020.106638
Medication adherence and rate of nicotine metabolism are associated with response to treatment with varenicline among smokers with HIV
Abstract
Introduction: PLWHA who smoke have shown lower cessation rates within placebo-controlled randomized trials of varenicline. Adherence and rate of nicotine metabolism may be associated with quit rates in such clinical trials.
Methods: This secondary analysis of a randomized placebo-controlled trial of varenicline for smoking among PLWHA (N = 179) examined the relationship between varenicline adherence (pill count, ≥80% of pills), nicotine metabolism (based on the nicotine metabolite ratio; NMR) and end-of-treatment smoking cessation (self-reported 7-day point prevalence abstinence, confirmed with carbon monoxide of ≤ 8 ppm, at the end of treatment; EOT).
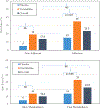
Results: Combining varenicline and placebo arms, greater adherence (OR = 1.011, 95% CI:1.00-1.02, p = 0.051) and faster nicotine metabolism (OR = 3.08, 95% CI:1.01-9.37, p = 0.047) were related to higher quit rates. In separate models, adherence (OR = 1.009, 95% CI:1.004-1.01, p < 0.001) and nicotine metabolism rate (OR = 2.04, 95% CI:1.19-3.49, p = 0.009) interacted with treatment arm to effect quit rates. The quit rate for varenicline vs. placebo was higher for both non-adherent (19% vs. 5%; χ2[1] = 2.80, p = 0.09) and adherent (35% vs. 15%; χ2[1] = 6.51, p = 0.01) participants, but the difference between treatment arms was statistically significant only for adherent participants. Likewise, among slow metabolizers (NMR < 0.31), the varenicline quit rate was not significantly higher vs. placebo (14% vs. 5%; χ2[1] = 1.17, p = 0.28) but, among fast metabolizers (NMR ≥ 0.31), the quit rate for varenicline was significantly higher vs. placebo (33% vs. 14%; χ2[1] = 4.43, p = 0.04).
Conclusions: Increasing varenicline adherence and ensuring that fast nicotine metabolizers receive varenicline may increase quit rates for PLWHA.
Keywords: Adherence; Anxiety; Depression; HIV; Smoking cessation; Varenicline.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest
Dr. Schnoll and Dr. Hitsman receive medication and placebo free from Pfizer and has provided consultation to Pfizer. Dr. Schnoll has provided consultation to GlaxoSmithKline and CuraLeaf. Dr. Gross serves on a Data and Safety Monitoring Board for a Pfizer drug unrelated to HIV or smoking.
Figures
References
-
- Anthenelli RM, Benowitz NL, West R, et al. (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet. 387(10037), 2507–2520. 10.1016/S0140-6736(16)30272-0. - DOI - PubMed
-
- Benard A, Tessier JF, Rambeloarisoa J, Bonnet F, Fossoux H, Neau D, et al. (2006). HIV infection and tobacco smoking behaviour: Prospects for prevention? ANRS CO3 Aquitaine Cohort, 2002. The International Journal of Tuberculosis and Lung Disease, 10(4), 378–383. - PubMed
-
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, & Miller IW (1998). Reliability and validity of a smoking timeline follow-back interview. Psychology Addiction Behaviour, 12(2), 101–112. 10.1037/0893-164X.12.2.101. - DOI
-
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, & Rapkin BD (2005). Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine & Tobacco Research, 7(4), 511–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical