Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques
- PMID: 32911637
- PMCID: PMC7571018
- DOI: 10.3390/molecules25184097
Stearic Acid/Layered Double Hydroxides Composite Thin Films Deposited by Combined Laser Techniques
Abstract
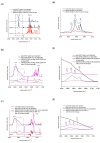
We report on the investigation of stearic acid-layered double hydroxide (LDH) composite films, with controlled wettability capabilities, deposited by a combined pulsed laser deposition (PLD)-matrix-assisted pulsed laser evaporation (MAPLE) system. Two pulsed lasers working in IR or UV were used for experiments, allowing the use of proper deposition parameters (wavelength, laser fluence, repetition rate) for each organic and inorganic component material. We have studied the time stability and wettability properties of the films and we have seen that the morphology of the surface has a low effect on the wettability of the surfaces. The obtained composite films consist in stearic acid aggregates in LDH structure, exhibiting a shift to hydrophobicity after 36 months of storage.
Keywords: laser deposition; layered double hydroxides; stearic acid; thin films.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Williams G.R., O’Hare D. Towards understanding, control and application of layered double hydroxide chemistry. J. Mater. Chem. 2006;16:3065–3074. doi: 10.1039/b604895a. - DOI
-
- Costantino U., Ambrogi V., Nocchetti M., Perioli L. Hydrotalcite-like compounds: Versatile layered hosts of molecular anions with biological activity. Micropor. Mesopor. Mat. 2008;107:149–160. doi: 10.1016/j.micromeso.2007.02.005. - DOI
-
- Forano C., Hibino T., Leroux F., Taviot-Guého C. Layered double hydroxides. In: Bergaya F., Theng B.K.G., Lagaly G., editors. Handbook of Clay Science. Elsevier Ltd.; Amsterdam, The Netherlands: 2006. pp. 1021–1095.
-
- Costantino U., Gallipoli A., Nocchetti M., Camino G., Bellucci F., Frache A. New nanocomposites constituted of polyethylene and organically modified ZnAl-hydrotalcites. Polym. Degrad. Stab. 2005;90:586–590. doi: 10.1016/j.polymdegradstab.2005.05.019. - DOI
-
- He F., Zhang L., Yang F., Chen L., Wu Q. New nanocomposites based on syndiotactic polystyrene and organo-modified ZnAl layered double hydroxide. J. Polym. Res. 2006;13:483–493. doi: 10.1007/s10965-006-9071-9. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

