Is There Evidence for IGF1R-Stimulating Abs in Graves' Orbitopathy Pathogenesis?
- PMID: 32911689
- PMCID: PMC7555308
- DOI: 10.3390/ijms21186561
Is There Evidence for IGF1R-Stimulating Abs in Graves' Orbitopathy Pathogenesis?
Abstract
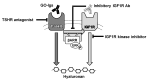
In this review, we summarize the evidence against direct stimulation of insulin-like growth factor 1 receptors (IGF1Rs) by autoantibodies in Graves' orbitopathy (GO) pathogenesis. We describe a model of thyroid-stimulating hormone (TSH) receptor (TSHR)/IGF1R crosstalk and present evidence that observations indicating IGF1R's role in GO could be explained by this mechanism. We evaluate the evidence for and against IGF1R as a direct target of stimulating IGF1R antibodies (IGF1RAbs) and conclude that GO pathogenesis does not involve directly stimulating IGF1RAbs. We further conclude that the preponderance of evidence supports TSHR as the direct and only target of stimulating autoantibodies in GO and maintain that the TSHR should remain a major target for further development of a medical therapy for GO in concert with drugs that target TSHR/IGF1R crosstalk.
Keywords: Graves’ orbital fibroblasts; Graves’ orbitopathy; IGF1R; IGF1R antibodies; TSHR; TSHR antibodies; hyaluronan; receptor crosstalk.
Conflict of interest statement
C.C.K., S.N., and M.C.G. have filed a patent pertaining to drug combinations targeting TSHR and IGF-1R.
Figures
Similar articles
-
TSH/IGF-1 Receptor Cross Talk in Graves' Ophthalmopathy Pathogenesis.J Clin Endocrinol Metab. 2016 Jun;101(6):2340-7. doi: 10.1210/jc.2016-1315. Epub 2016 Apr 4. J Clin Endocrinol Metab. 2016. PMID: 27043163 Free PMC article.
-
PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves' ophthalmopathy patients.J Clin Endocrinol Metab. 2012 Jun;97(6):E944-53. doi: 10.1210/jc.2012-1020. Epub 2012 Mar 21. J Clin Endocrinol Metab. 2012. PMID: 22438231
-
Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy.Exp Eye Res. 2016 Jan;142:83-91. doi: 10.1016/j.exer.2015.02.007. Exp Eye Res. 2016. PMID: 26675405 Review.
-
Orbital Signaling in Graves' Orbitopathy.Front Endocrinol (Lausanne). 2021 Nov 9;12:739994. doi: 10.3389/fendo.2021.739994. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34899596 Free PMC article. Review.
-
Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves' orbitopathy.Best Pract Res Clin Endocrinol Metab. 2012 Jun;26(3):291-302. doi: 10.1016/j.beem.2011.10.002. Best Pract Res Clin Endocrinol Metab. 2012. PMID: 22632366 Free PMC article. Review.
Cited by
-
Modulating TSH Receptor Signaling for Therapeutic Benefit.Eur Thyroid J. 2020 Dec;9(Suppl 1):66-77. doi: 10.1159/000511871. Epub 2020 Nov 23. Eur Thyroid J. 2020. PMID: 33511087 Free PMC article.
-
Research progress on the pathogenesis of Graves' ophthalmopathy: Based on immunity, noncoding RNA and exosomes.Front Immunol. 2022 Aug 23;13:952954. doi: 10.3389/fimmu.2022.952954. eCollection 2022. Front Immunol. 2022. PMID: 36081502 Free PMC article. Review.
-
Graves' Autoantibodies Exhibit Different Stimulating Activities in Cultures of Thyrocytes and Orbital Fibroblasts Not Reflected by Clinical Assays.Thyroid. 2022 Jan;32(1):90-96. doi: 10.1089/thy.2021.0326. Epub 2021 Nov 29. Thyroid. 2022. PMID: 34714162 Free PMC article.
-
Thinking inside the box: Current insights into targeting orbital tissue remodeling and inflammation in thyroid eye disease.Surv Ophthalmol. 2022 May-Jun;67(3):858-874. doi: 10.1016/j.survophthal.2021.08.010. Epub 2021 Sep 4. Surv Ophthalmol. 2022. PMID: 34487739 Free PMC article. Review.
-
A review of TSHR- and IGF-1R-related pathogenesis and treatment of Graves' orbitopathy.Front Immunol. 2023 Jan 19;14:1062045. doi: 10.3389/fimmu.2023.1062045. eCollection 2023. Front Immunol. 2023. PMID: 36742308 Free PMC article. Review.
References
-
- Rubin R., Baserga R. Insulin-like growth factor-I receptor. Its role in cell proliferation, apoptosis, and tumorigenicity. Lab. Investig. 1995;73:311–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

