Mayaro Virus Pathogenesis and Transmission Mechanisms
- PMID: 32911824
- PMCID: PMC7558846
- DOI: 10.3390/pathogens9090738
Mayaro Virus Pathogenesis and Transmission Mechanisms
Abstract
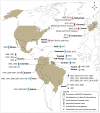
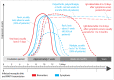
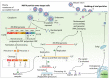
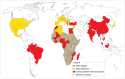
Mayaro virus (MAYV), isolated for the first time in Trinidad and Tobago, has captured the attention of public health authorities worldwide following recent outbreaks in the Americas. It has a propensity to be exported outside its original geographical range, because of the vast distribution of its vectors. Moreover, most of the world population is immunologically naïve with respect to infection with MAYV which makes this virus a true threat. The recent invasion of several countries by Aedesalbopictus underscores the risk of potential urban transmission of MAYV in both tropical and temperate regions. In humans, the clinical manifestations of MAYV disease range from mild fever, rash, and joint pain to arthralgia. In the absence of a licensed vaccine and clinically proven therapeutics against Mayaro fever, prevention focuses mainly on household mosquito control. However, as demonstrated for other arboviruses, mosquito control is rather inefficient for outbreak management and alternative approaches to contain the spread of MAYV are therefore necessary. Despite its strong epidemic potential, little is currently known about MAYV. This review addresses various aspects of MAYV, including its epidemiology, vector biology, mode of transmission, and clinical complications, as well as the latest developments in MAYV diagnosis.
Keywords: Aedes; Mayaro; Togaviridae; alphavirus; emerging arbovirus; vector competence.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

