Azithromycin ameliorates sulfur dioxide-induced airway epithelial damage and inflammatory responses
- PMID: 32912304
- PMCID: PMC7488110
- DOI: 10.1186/s12931-020-01489-8
Azithromycin ameliorates sulfur dioxide-induced airway epithelial damage and inflammatory responses
Abstract
Background: The airway epithelium (AE) forms the first line of defence against harmful particles and pathogens. Barrier failure of the airway epithelium contributes to exacerbations of a range of lung diseases that are commonly treated with Azithromycin (AZM). In addition to its anti-bacterial function, AZM has immunomodulatory effects which are proposed to contribute to its clinical effectiveness. In vitro studies have shown the AE barrier-enhancing effects of AZM. The aim of this study was to analyze whether AE damage caused by inhalation of sulfur dioxide (SO2) in a murine model could be reduced by pre-treatment with AZM.
Methods: The leakiness of the AE barrier was evaluated after SO2 exposure by measuring levels of human serum albumin (HSA) in bronchoalveolar lavage fluid (BALF). Protein composition in BALF was also assessed and lung tissues were evaluated across treatments using histology and gene expression analysis.
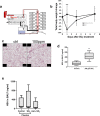
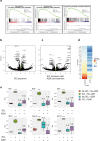
Results: AZM pre-treatment (2 mg/kg p.o. 5 times/week for 2 weeks) resulted in reduced glutathione-S-transferases in BALF of SO2 injured mice compared to control (without AZM treatment). AZM treated mice had increased intracellular vacuolization including lamellar bodies and a reduction in epithelial shedding after injury in addition to a dampened SO2-induced inflammatory response.
Conclusions: Using a mouse model of AE barrier dysfunction we provide evidence for the protective effects of AZM in vivo, possibly through stabilizing the intracellular microenvironment and reducing inflammatory responses. Our data provide insight into the mechanisms contributing to the efficacy of AZM in the treatment of airway diseases.
Keywords: Azithromycin; Glutathione-S-transferase; Immunomodulation; Lamellar bodies; Lung barrier enhancement.
Conflict of interest statement
All authors have declared that they work for and / or are shareholders in EpiEndo Pharmaceuticals.
Figures


Similar articles
-
Azithromycin has lung barrier protective effects in a cell model mimicking ventilator-induced lung injury.ALTEX. 2020;37(4):545-560. doi: 10.14573/altex.2001271. Epub 2020 May 19. ALTEX. 2020. PMID: 32449787
-
Azithromycin induces epidermal differentiation and multivesicular bodies in airway epithelia.Respir Res. 2019 Jun 24;20(1):129. doi: 10.1186/s12931-019-1101-3. Respir Res. 2019. PMID: 31234850 Free PMC article.
-
Effects of azithromycin on glutathione S-transferases in cystic fibrosis airway cells.Am J Respir Cell Mol Biol. 2009 Aug;41(2):199-206. doi: 10.1165/rcmb.2008-0013OC. Epub 2008 Dec 18. Am J Respir Cell Mol Biol. 2009. PMID: 19097986
-
Mechanism of azithromycin in airway diseases.J Int Med Res. 2020 Jun;48(6):300060520932104. doi: 10.1177/0300060520932104. J Int Med Res. 2020. PMID: 32589092 Free PMC article. Review.
-
Nonantimicrobial Actions of Macrolides: Overview and Perspectives for Future Development.Pharmacol Rev. 2021 Oct;73(4):233-262. doi: 10.1124/pharmrev.121.000300. Pharmacol Rev. 2021. PMID: 34716226 Review.
Cited by
-
Azithromycin ameliorated cigarette smoke-induced airway epithelial barrier dysfunction by activating Nrf2/GCL/GSH signaling pathway.Respir Res. 2023 Mar 6;24(1):69. doi: 10.1186/s12931-023-02375-9. Respir Res. 2023. PMID: 36879222 Free PMC article.
-
Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD.Eur Respir Rev. 2022 Mar 23;31(163):210112. doi: 10.1183/16000617.0112-2021. Print 2022 Mar 31. Eur Respir Rev. 2022. PMID: 35321933 Free PMC article. Review.
-
Air Pollutants Associated Hospitalization in Pediatric Pneumonia: A National Database Analysis.Pediatr Pulmonol. 2025 Feb;60(2):e71009. doi: 10.1002/ppul.71009. Pediatr Pulmonol. 2025. PMID: 39950392 Free PMC article.
-
Association Between the Ambient Fine Particulate Pollution and the Daily Internal Medicine Outpatient Visits in Zhoushan, China: A Time-Series Study.Front Public Health. 2021 Oct 26;9:749191. doi: 10.3389/fpubh.2021.749191. eCollection 2021. Front Public Health. 2021. PMID: 34765582 Free PMC article.
-
Sulfur dioxide exposure of mice induces peribronchiolar fibrosis-A defining feature of deployment-related constrictive bronchiolitis.PLoS One. 2025 Jan 24;20(1):e0313992. doi: 10.1371/journal.pone.0313992. eCollection 2025. PLoS One. 2025. PMID: 39854594 Free PMC article.
References
-
- Agency E-USPE. Integrated science assessment for sulfur oxide - health criteria. 2008.
-
- Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, Perez-Padilla R, Rice MB, Riojas-Rodriguez H, Sood A, et al. Air pollution and noncommunicable diseases: a review by the forum of international respiratory Societies' environmental committee, part 2: air pollution and organ systems. Chest. 2019;155:417–426. doi: 10.1016/j.chest.2018.10.041. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

