Association between fetal sex and maternal plasma microRNA responses to prenatal alcohol exposure: evidence from a birth outcome-stratified cohort
- PMID: 32912312
- PMCID: PMC7488011
- DOI: 10.1186/s13293-020-00327-2
Association between fetal sex and maternal plasma microRNA responses to prenatal alcohol exposure: evidence from a birth outcome-stratified cohort
Abstract
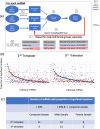
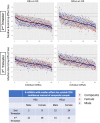
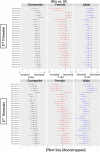
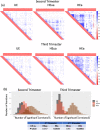
Most persons with fetal alcohol spectrum disorders (FASDs) remain undiagnosed or are diagnosed in later life. To address the need for earlier diagnosis, we previously assessed miRNAs in the blood plasma of pregnant women who were classified as unexposed to alcohol (UE), heavily exposed with affected infants (HEa), or heavily exposed with apparently unaffected infants (HEua). We reported that maternal miRNAs predicted FASD-related growth and psychomotor deficits in infants. Here, we assessed whether fetal sex influenced alterations in maternal circulating miRNAs following prenatal alcohol exposure (PAE). To overcome the loss of statistical power due to disaggregating maternal samples by fetal sex, we adapted a strategy of iterative bootstrap resampling with replacement to assess the stability of statistical parameter estimates. Bootstrap estimates of parametric and effect size tests identified male and female fetal sex-associated maternal miRNA responses to PAE that were not observed in the aggregated sample. Additionally, we observed, in HEa mothers of female, but not male fetuses, a network of co-secreted miRNAs whose expression was linked to miRNAs encoded on the X-chromosome. Interestingly, the number of significant miRNA correlations for the HEua group mothers with female fetuses was intermediate between HEa and UE mothers at mid-pregnancy, but more similar to UE mothers by the end of pregnancy. Collectively, these data show that fetal sex predicts maternal circulating miRNA adaptations, a critical consideration when adopting maternal miRNAs as diagnostic biomarkers. Moreover, a maternal co-secretion network, predominantly in pregnancies with female fetuses, emerged as an index of risk for adverse birth outcomes due to PAE.
Keywords: Bootstrap resampling; Extracellular miRNAs; Fetal alcohol spectrum disorders; Maternal miRNA co-secretion; Sex as a biological variable.
Conflict of interest statement
The authors declare that they have no competing interests
Figures


Similar articles
-
Plasma miRNA Profiles in Pregnant Women Predict Infant Outcomes following Prenatal Alcohol Exposure.PLoS One. 2016 Nov 9;11(11):e0165081. doi: 10.1371/journal.pone.0165081. eCollection 2016. PLoS One. 2016. PMID: 27828986 Free PMC article.
-
Fetal Brain-Derived Exosomal miRNAs from Maternal Blood: Potential Diagnostic Biomarkers for Fetal Alcohol Spectrum Disorders (FASDs).Int J Mol Sci. 2024 May 27;25(11):5826. doi: 10.3390/ijms25115826. Int J Mol Sci. 2024. PMID: 38892014 Free PMC article.
-
Maternal and neonatal plasma microRNA biomarkers for fetal alcohol exposure in an ovine model.Alcohol Clin Exp Res. 2014 May;38(5):1390-400. doi: 10.1111/acer.12378. Epub 2014 Mar 3. Alcohol Clin Exp Res. 2014. PMID: 24588274 Free PMC article.
-
Maternal iron nutriture as a critical modulator of fetal alcohol spectrum disorder risk in alcohol-exposed pregnancies.Biochem Cell Biol. 2018 Apr;96(2):204-212. doi: 10.1139/bcb-2017-0206. Epub 2017 Oct 10. Biochem Cell Biol. 2018. PMID: 29017023 Free PMC article. Review.
-
Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn.J Perinatol. 2012 Sep;32(9):652-9. doi: 10.1038/jp.2012.57. Epub 2012 May 17. J Perinatol. 2012. PMID: 22595965 Review.
Cited by
-
Objective assessment of alcohol consumption in early pregnancy using phosphatidylethanol: a cross-sectional study.BMC Pregnancy Childbirth. 2021 Apr 30;21(1):342. doi: 10.1186/s12884-021-03804-7. BMC Pregnancy Childbirth. 2021. PMID: 33931032 Free PMC article.
-
Maternal circulating miRNAs contribute to negative pregnancy outcomes by altering placental transcriptome and fetal vascular dynamics.PLoS One. 2023 Nov 6;18(11):e0290720. doi: 10.1371/journal.pone.0290720. eCollection 2023. PLoS One. 2023. PMID: 37930978 Free PMC article.
-
Microbiota and nutrition as risk and resiliency factors following prenatal alcohol exposure.Front Neurosci. 2023 Jun 15;17:1182635. doi: 10.3389/fnins.2023.1182635. eCollection 2023. Front Neurosci. 2023. PMID: 37397440 Free PMC article. Review.
-
Prenatal opioid-exposed infant extracellular miRNA signature obtained at birth predicts severity of neonatal opioid withdrawal syndrome.Sci Rep. 2022 Apr 8;12(1):5941. doi: 10.1038/s41598-022-09793-7. Sci Rep. 2022. PMID: 35396369 Free PMC article.
-
Recent Advances in the Role of Non-coding RNAs in Fetal Alcohol Spectrum Disorders.Adv Exp Med Biol. 2025;1473:129-155. doi: 10.1007/978-3-031-81908-7_7. Adv Exp Med Biol. 2025. PMID: 40128478 Review.
References
-
- Bakhireva LN, Garrison L, Shrestha S, Sharkis J, Miranda R, Rogers K. Challenges of diagnosing fetal alcohol spectrum disorders in foster and adopted children. Alcohol. 2017;67:37–43. - PubMed
-
- Bakhireva LN, Schatz M, Jones KL, Tucker CM, Slymen DJ, Klonoff-Cohen HS, Gresham L, Johnson D, Chambers CD, Group, O.C.R Fetal sex and maternal asthma control in pregnancy. J Asthma. 2008;45:403–407. - PubMed
-
- Bakhireva LN, Sharkis J, Shrestha S, Miranda-Sohrabji TJ, Williams S, Miranda RC. Prevalence of prenatal alcohol exposure in the State of Texas as assessed by phosphatidylethanol in newborn dried blood spot specimens. Alcohol Clin Exp Res. 2017;41:1004–1011. - PubMed

