Chromonomer: A Tool Set for Repairing and Enhancing Assembled Genomes Through Integration of Genetic Maps and Conserved Synteny
- PMID: 32912931
- PMCID: PMC7642942
- DOI: 10.1534/g3.120.401485
Chromonomer: A Tool Set for Repairing and Enhancing Assembled Genomes Through Integration of Genetic Maps and Conserved Synteny
Abstract
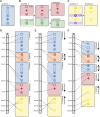
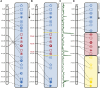
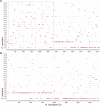
The pace of the sequencing and computational assembly of novel reference genomes is accelerating. Though DNA sequencing technologies and assembly software tools continue to improve, biological features of genomes such as repetitive sequence as well as molecular artifacts that often accompany sequencing library preparation can lead to fragmented or chimeric assemblies. If left uncorrected, defects like these trammel progress on understanding genome structure and function, or worse, positively mislead this research. Fortunately, integration of additional, independent streams of information, such as a marker-dense genetic map and conserved orthologous gene order from related taxa, can be used to scaffold together unlinked, disordered fragments and to restructure a reference genome where it is incorrectly joined. We present a tool set for automating these processes, one that additionally tracks any changes to the assembly and to the genetic map, and which allows the user to scrutinize these changes with the help of web-based, graphical visualizations. Chromonomer takes a user-defined reference genome, a map of genetic markers, and, optionally, conserved synteny information to construct an improved reference genome of chromosome models: a "chromonome". We demonstrate Chromonomer's performance on genome assemblies and genetic maps that have disparate characteristics and levels of quality.
Keywords: Genome assembly; RADseq; conserved synteny; genetic map.
Copyright © 2020 Catchen et al.
Figures






References
-
- Bednar J., and Watt T., 1984. Alpha-trimmed means and their relationship to median filters. IEEE Trans. Acoust. Speech Signal Process. 32: 145–153. 10.1109/TASSP.1984.1164279 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
