Yorkshire Lung Screening Trial (YLST): protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk
- PMID: 32912947
- PMCID: PMC7485242
- DOI: 10.1136/bmjopen-2020-037075
Yorkshire Lung Screening Trial (YLST): protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk
Abstract
Introduction: Lung cancer is the world's leading cause of cancer death. Low-dose computed tomography (LDCT) screening reduced lung cancer mortality by 20% in the US National Lung Screening Trial. Here, we present the Yorkshire Lung Screening Trial (YLST), which will address key questions of relevance for screening implementation.
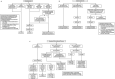
Methods and analysis: Using a single-consent Zelen's design, ever-smokers aged 55-80 years registered with a general practice in Leeds will be randomised (1:1) to invitation to a telephone-based risk-assessment for a Lung Health Check or to usual care. The anticipated number randomised by household is 62 980 individuals. Responders at high risk will be invited for LDCT scanning for lung cancer on a mobile van in the community. There will be two rounds of screening at an interval of 2 years. Primary objectives are (1) measure participation rates, (2) compare the performance of PLCOM2012 (threshold ≥1.51%), Liverpool Lung Project (V.2) (threshold ≥5%) and US Preventive Services Task Force eligibility criteria for screening population selection and (3) assess lung cancer outcomes in the intervention and usual care arms. Secondary evaluations include health economics, quality of life, smoking rates according to intervention arm, screening programme performance with ancillary biomarker and smoking cessation studies.
Ethics and dissemination: The study has been approved by the Greater Manchester West research ethics committee (18-NW-0012) and the Health Research Authority following review by the Confidentiality Advisory Group. The results will be disseminated through publication in peer-reviewed scientific journals, presentation at conferences and on the YLST website.
Trial registration numbers: ISRCTN42704678 and NCT03750110.
Keywords: chest imaging; respiratory tract tumours.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: PAJC has received consultation fees and shares options from Everest Detection. PAJC is supported by the NIHR Manchester Biomedical Research Centre.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
