Yorkshire Enhanced Stop Smoking (YESS) study: a protocol for a randomised controlled trial to evaluate the effect of adding a personalised smoking cessation intervention to a lung cancer screening programme
- PMID: 32912948
- PMCID: PMC7485260
- DOI: 10.1136/bmjopen-2020-037086
Yorkshire Enhanced Stop Smoking (YESS) study: a protocol for a randomised controlled trial to evaluate the effect of adding a personalised smoking cessation intervention to a lung cancer screening programme
Abstract
Introduction: Integration of smoking cessation (SC) into lung cancer screening is essential to optimise clinical and cost effectiveness. The most effective way to use this 'teachable moment' is unclear. The Yorkshire Enhanced Stop Smoking study will measure the effectiveness of an SC service integrated within the Yorkshire Lung Screening Trial (YLST) and will test the efficacy of a personalised SC intervention, incorporating incidental findings detected on the low-dose CT scan performed as part of YLST.
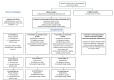
Methods and analysis: Unless explicitly declined, all smokers enrolled in YLST will see an SC practitioner at baseline and receive SC support over 4 weeks comprising behavioural support, pharmacotherapy and/or a commercially available e-cigarette. Eligible smokers will be randomised (1:1 in permuted blocks of random size up to size 6) to receive either an enhanced, personalised SC support package, including CT scan images, or continued standard best practice. Anticipated recruitment is 1040 smokers (January 2019-December 2020). The primary objective is to measure 7-day point prevalent carbon monoxide (CO) validated SC after 3 months. Secondary outcomes include CO validated cessation at 4 weeks and 12 months, self-reported continuous cessation at 4 weeks, 3 months and 12 months, attempts to quit smoking and changes in psychological variables, including perceived risk of lung cancer, motivation to quit smoking tobacco, confidence and efficacy beliefs (self and response) at all follow-up points. A process evaluation will explore under which circumstances and on which groups the intervention works best, test intervention fidelity and theory test the mechanisms of intervention impact.
Ethics and dissemination: This study has been approved by the East Midlands-Derby Research Ethics Committee (18/EM/0199) and the Health Research Authority/Health and Care Research Wales. Results will be disseminated through publication in peer-reviewed scientific journals, presentation at conferences and via the YLST website.
Trial registration numbers: ISRCTN63825779, NCT03750110.
Keywords: CT; protocols & guidelines; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: PAJC has received consultation fees and share options from Everest Detection.
Figures

References
-
- Cancer Research UK Cancer incidence for common cancers. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/i... [Accessed 10 Oct 2019].
-
- Office for National Statistics Smoking (General lifestyle survey overview - a report on the 2011 general lifestyle survey), 2013.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
