PERSonalised Incentives for Supporting Tobacco cessation (PERSIST) among healthcare employees: a randomised controlled trial protocol
- PMID: 32912952
- PMCID: PMC7482494
- DOI: 10.1136/bmjopen-2020-037799
PERSonalised Incentives for Supporting Tobacco cessation (PERSIST) among healthcare employees: a randomised controlled trial protocol
Abstract
Background: Smoking is the primary preventable risk factor for disease and premature mortality. It is highly addictive and cessation attempts are often unsuccessful. Incentive-based programmes may be an effective method to reach sustained abstinence. Individualisation of incentives based on personal characteristics yields potential to further increase the effectiveness of incentive-based programmes.
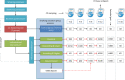
Method: A randomised controlled trial among healthcare workers recruited through their employer and signed up for a group-based smoking cessation programme. The intervention under study is the provision of personalised incentives on validated smoking cessation at several time points after the smoking cessation programme. A total of 220 participants are required. Participants are randomised 1:1 into intervention (personalised incentives) or control (no incentives). All participants join the group-based programme. Incentives are provided on validated abstinence directly after the smoking cessation programme and after 3, 6 and 12 months.Incentives are provided according to four schemes:(1) Standard: total reward size €350, pay-out scheme: €50 (t=0), €50 (t=3 months), €50 (t=6 months) and €200 (t=12 months), (2) descending: total reward size €300, pay-out scheme: €150, €100, €50 and €0, (3) ascending: total reward size: €400, pay-out scheme: €0, €0, €50 and €350 and (4) deposit: total reward size €450, pay-out scheme: €50, €50, €150, €200; participants pay a €100 deposit, returned conditional on abstinence after 6 months.Advice on which incentive scheme suits participants best is based on willingness to provide a deposit, readiness to quit, nicotine dependency and long-term or short-term reward preference. Participants are free to deviate from this advice. Abstinence is validated at each time point, with 15 months of total follow-up. The primary end point is validated abstinence at 12 months. Effectiveness will be determined by intention-to-treat analysis.
Ethics and dissemination: The Erasmus MC Medical Ethics Committee decided that according to the Dutch Human Research Law (WMO), the protocol required no formal ethical approval. The results will be published in a peer-reviewed scientific journal and communicated to the participants.
Trial registration number: Netherlands Trial Register NL7711.
Keywords: validated.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Forouzanfar MH, Afshin A, Alexander LT, et al. . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016;388:1659–724. 10.1016/S0140-6736(16)31679-8 - DOI - PMC - PubMed
-
- Netherlands S Leefstijl en (preventief) gezondheidsonderzoek; persoonskenmerken, 2019.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
