Cardiac phenotype in ATP1A3-related syndromes: A multicenter cohort study
- PMID: 32913013
- PMCID: PMC7734736
- DOI: 10.1212/WNL.0000000000010794
Cardiac phenotype in ATP1A3-related syndromes: A multicenter cohort study
Abstract
Objective: To define the risks and consequences of cardiac abnormalities in ATP1A3-related syndromes.
Methods: Patients meeting clinical diagnostic criteria for rapid-onset dystonia-parkinsonism (RDP), alternating hemiplegia of childhood (AHC), and cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) with ATP1A3 genetic analysis and at least 1 cardiac assessment were included. We evaluated the cardiac phenotype in an Atp1a3 knock-in mouse (Mashl+/-) to determine the sequence of events in seizure-related cardiac death.
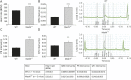
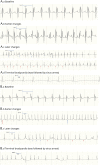
Results: Ninety-eight patients with AHC, 9 with RDP, and 3 with CAPOS (63 female, mean age 17 years) were included. Resting ECG abnormalities were found in 52 of 87 (60%) with AHC, 2 of 3 (67%) with CAPOS, and 6 of 9 (67%) with RDP. Serial ECGs showed dynamic changes in 10 of 18 patients with AHC. The first Holter ECG was abnormal in 24 of 65 (37%) cases with AHC and RDP with either repolarization or conduction abnormalities. Echocardiography was normal. Cardiac intervention was required in 3 of 98 (≈3%) patients with AHC. In the mouse model, resting ECGs showed intracardiac conduction delay; during induced seizures, heart block or complete sinus arrest led to death.
Conclusions: We found increased prevalence of ECG dynamic abnormalities in all ATP1A3-related syndromes, with a risk of life-threatening cardiac rhythm abnormalities equivalent to that in established cardiac channelopathies (≈3%). Sudden cardiac death due to conduction abnormality emerged as a seizure-related outcome in murine Atp1a3-related disease. ATP1A3-related syndromes are cardiac diseases and neurologic diseases. We provide guidance to identify patients potentially at higher risk of sudden cardiac death who may benefit from insertion of a pacemaker or implantable cardioverter-defibrillator.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, et al. . Mutations in the Na(+)/K(+)-ATPase alpha-3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 2004;43:169–175. - PubMed
-
- Brashear A, Sweadner KJ, Cook JF, Swoboda KJ, Ozelius L. ATP1A3-Related neurologic disorders. 2008. In: Adam MP, Ardinger, HH, Pagon RA, et al.., editors. GeneReviews® [Internet]. Seattle: University of Washington; 1993–2018. Available at: ncbi.nlm.nih.gov/books/NBK1115/. Accessed January 5, 2019. - PubMed
-
- Masoud M, Prange L, Wuchich J, et al. . Diagnosis and treatment of alternating hemiplegia of childhood. Curr Treat Options Neurol 2017;19:8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
