SMALL ORGAN4 Is a Ribosome Biogenesis Factor Involved in 5.8S Ribosomal RNA Maturation
- PMID: 32913045
- PMCID: PMC7723108
- DOI: 10.1104/pp.19.01540
SMALL ORGAN4 Is a Ribosome Biogenesis Factor Involved in 5.8S Ribosomal RNA Maturation
Abstract
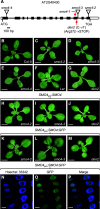
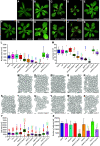
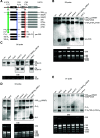
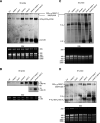
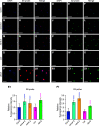
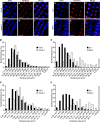
Ribosome biogenesis is crucial for cellular metabolism and has important implications for disease and aging. Human (Homo sapiens) glioma tumor-suppressor candidate region gene2 (GLTSCR2) and yeast (Saccharomyces cerevisiae) Nucleolar protein53 (Nop53) are orthologous proteins with demonstrated roles as ribosome biogenesis factors; knockdown of GLTSCR2 impairs maturation of 18S and 5.8S ribosomal RNAs (rRNAs), and Nop53 is required for maturation of 5.8S and 25S rRNAs. Here, we characterized SMALL ORGAN4 (SMO4), the most likely ortholog of human GLTSCR2 and yeast Nop53 in Arabidopsis (Arabidopsis thaliana). Loss of function of SMO4 results in a mild morphological phenotype; however, we found that smo4 mutants exhibit strong cytological and molecular phenotypes: nucleolar hypertrophy and disorganization, overaccumulation of 5.8S and 18S rRNA precursors, and an imbalanced 40S:60S ribosome subunit ratio. Like yeast Nop53 and human GLTSCR2, Arabidopsis SMO4 participates in 5.8S rRNA maturation. In yeast, Nop53 cooperates with mRNA transport4 (Mtr4) for 5.8S rRNA maturation. In Arabidopsis, we found that SMO4 plays similar roles in the 5.8S rRNA maturation pathway than those described for MTR4. However, SMO4 seems not to participate in the degradation of by-products derived from the 5'-external transcribed spacer (ETS) of 45S pre-rRNA, as MTR4 does.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures


Comment in
-
How to Make an Extraordinary Machine: SMALL ORGAN4 Regulates Ribosome Biogenesis in Plants.Plant Physiol. 2020 Dec;184(4):1627-1629. doi: 10.1104/pp.20.01456. Plant Physiol. 2020. PMID: 33277331 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

