Glomerular clusterin expression is increased in diabetic nephropathy and protects against oxidative stress-induced apoptosis in podocytes
- PMID: 32913257
- PMCID: PMC7484791
- DOI: 10.1038/s41598-020-71629-z
Glomerular clusterin expression is increased in diabetic nephropathy and protects against oxidative stress-induced apoptosis in podocytes
Abstract
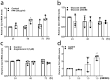
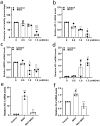
Clusterin, a glycoprotein encoded by the CLU gene, is expressed in many tissues, including the kidney, and clusterin expression is upregulated in the glomeruli of patients with various forms of kidney disease. Here, we investigated the role of clusterin in diabetic nephropathy (DN). In this study, we found that glomerular clusterin expression was increased in both patients with DN and streptozotocin-induced diabetic mice and that it co-localised with the podocyte marker WT1, indicating clusterin is expressed in podocytes. In our in vitro analysis, we found no significant change in CLU mRNA expression in podocytes following stimulation with high glucose and angiotensin II; in contrast, CLU mRNA expression was significantly upregulated following methylglyoxal stimulation. Methylglyoxal treatment also significantly decreased the mRNA expression of the slit diaphragm markers ZO-1 and NEPH1 and significantly increased the mRNA expression of the oxidative stress marker HO-1. Lastly, we showed that pre-incubating podocytes with recombinant human clusterin protein increased podocyte survival, prevented slit diaphragm damage, and reduced oxidative stress‒induced apoptosis following methylglyoxal stimulation. Taken together, our results indicate that glomerular clusterin is upregulated in DN, and this increase in clusterin expression may protect against oxidative stress-induced apoptosis in podocytes, providing a possible new therapeutic target for DN and other kidney diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Protection of CTGF Antibody Against Diabetic Nephropathy in Mice Via Reducing Glomerular β-Catenin Expression and Podocyte Epithelial-Mesenchymal Transition.J Cell Biochem. 2017 Nov;118(11):3706-3712. doi: 10.1002/jcb.26017. Epub 2017 May 16. J Cell Biochem. 2017. PMID: 28370212
-
High glucose provokes microvesicles generation from glomerular podocytes via NOX4/ROS pathway.Biosci Rep. 2019 Nov 29;39(11):BSR20192554. doi: 10.1042/BSR20192554. Biosci Rep. 2019. PMID: 31664454 Free PMC article.
-
Apoc1 Knockdown Alleviates High Glucose-induced Oxidative Stress and Apoptosis of Renal Tubular Cells by Binding to Clusterin.Cell Biochem Biophys. 2025 Jun;83(2):2253-2263. doi: 10.1007/s12013-024-01636-8. Epub 2024 Dec 4. Cell Biochem Biophys. 2025. PMID: 39630345
-
Diabetic Nephropathy: Perspective on Novel Molecular Mechanisms.Trends Endocrinol Metab. 2016 Nov;27(11):820-830. doi: 10.1016/j.tem.2016.07.002. Epub 2016 Jul 25. Trends Endocrinol Metab. 2016. PMID: 27470431 Review.
-
Glomerular podocytes in diabetic renal disease.Adv Clin Exp Med. 2019 Dec;28(12):1711-1715. doi: 10.17219/acem/104534. Adv Clin Exp Med. 2019. PMID: 31851794 Review.
Cited by
-
Hydroxyurea Mitigates Heme-Induced Inflammation and Kidney Injury in Humanized Sickle Cell Mice.Int J Mol Sci. 2025 Mar 30;26(7):3214. doi: 10.3390/ijms26073214. Int J Mol Sci. 2025. PMID: 40244015 Free PMC article.
-
Inulin Reduces Kidney Damage in Type 2 Diabetic Mice by Decreasing Inflammation and Serum Metabolomics.J Diabetes Res. 2024 May 2;2024:1222395. doi: 10.1155/2024/1222395. eCollection 2024. J Diabetes Res. 2024. PMID: 38725443 Free PMC article.
-
Clusterin-carrying extracellular vesicles derived from human umbilical cord mesenchymal stem cells restore the ovarian function of premature ovarian failure mice through activating the PI3K/AKT pathway.Stem Cell Res Ther. 2024 Sep 13;15(1):300. doi: 10.1186/s13287-024-03926-7. Stem Cell Res Ther. 2024. PMID: 39272156 Free PMC article.
-
Biomarkers in Contrast-Induced Nephropathy: Advances in Early Detection, Risk Assessment, and Prevention Strategies.Int J Mol Sci. 2025 Mar 21;26(7):2869. doi: 10.3390/ijms26072869. Int J Mol Sci. 2025. PMID: 40243457 Free PMC article. Review.
-
N6-Methyladenosine Methyltransferase METTL3 Alleviates Diabetes-Induced Testicular Damage through Modulating TUG1/Clusterin Axis.Diabetes Metab J. 2023 Mar;47(2):287-300. doi: 10.4093/dmj.2021.0306. Epub 2023 Jan 19. Diabetes Metab J. 2023. PMID: 36653890 Free PMC article.
References
-
- de Silva HV, et al. A 70-kDa apolipoprotein designated ApoJ is a marker for subclasses of human plasma high density lipoproteins. J. Biol. Chem. 1990;265:13240–13247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

