Understanding diaschisis models of attention dysfunction with rTMS
- PMID: 32913263
- PMCID: PMC7483730
- DOI: 10.1038/s41598-020-71692-6
Understanding diaschisis models of attention dysfunction with rTMS
Abstract
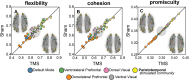
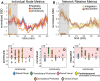
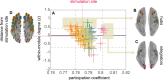
Visual attentive tracking requires a balance of excitation and inhibition across large-scale frontoparietal cortical networks. Using methods borrowed from network science, we characterize the induced changes in network dynamics following low frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) as an inhibitory noninvasive brain stimulation protocol delivered over the intraparietal sulcus. When participants engaged in visual tracking, we observed a highly stable network configuration of six distinct communities, each with characteristic properties in node dynamics. Stimulation to parietal cortex had no significant impact on the dynamics of the parietal community, which already exhibited increased flexibility and promiscuity relative to the other communities. The impact of rTMS, however, was apparent distal from the stimulation site in lateral prefrontal cortex. rTMS temporarily induced stronger allegiance within and between nodal motifs (increased recruitment and integration) in dorsolateral and ventrolateral prefrontal cortex, which returned to baseline levels within 15 min. These findings illustrate the distributed nature by which inhibitory rTMS perturbs network communities and is preliminary evidence for downstream cortical interactions when using noninvasive brain stimulation for behavioral augmentations.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Local Immediate versus Long-Range Delayed Changes in Functional Connectivity Following rTMS on the Visual Attention Network.Brain Stimul. 2017 Mar-Apr;10(2):263-269. doi: 10.1016/j.brs.2016.10.009. Epub 2016 Oct 19. Brain Stimul. 2017. PMID: 27838275 Free PMC article.
-
Attention Networks in the Parietooccipital Cortex Modulate Activity of the Human Vestibular Cortex during Attentive Visual Processing.J Neurosci. 2020 Jan 29;40(5):1110-1119. doi: 10.1523/JNEUROSCI.1952-19.2019. Epub 2019 Dec 9. J Neurosci. 2020. PMID: 31818978 Free PMC article.
-
Changes of oscillatory brain activity induced by repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in healthy subjects.Neuroimage. 2014 Mar;88:91-9. doi: 10.1016/j.neuroimage.2013.11.029. Epub 2013 Nov 21. Neuroimage. 2014. PMID: 24269574
-
Prefrontal and parietal cortex in human episodic memory: an interference study by repetitive transcranial magnetic stimulation.Eur J Neurosci. 2006 Feb;23(3):793-800. doi: 10.1111/j.1460-9568.2006.04600.x. Eur J Neurosci. 2006. PMID: 16487159
-
[Biological correlates of prefrontal activating and temporoparietal inhibiting treatment with repetitive transcranial magnetic stimulation (rTMS)].Fortschr Neurol Psychiatr. 2009 Aug;77(8):432-43. doi: 10.1055/s-0028-1109494. Epub 2009 Jun 16. Fortschr Neurol Psychiatr. 2009. PMID: 19533575 Review. German.
Cited by
-
A Discussion of a Case of Paradoxical Ipsilateral Hemiparesis in a Patient Diagnosed with Pterional Meningioma.J Clin Med. 2025 Apr 15;14(8):2689. doi: 10.3390/jcm14082689. J Clin Med. 2025. PMID: 40283519 Free PMC article.
-
Advancing working memory research through cortico-cortical transcranial magnetic stimulation.Front Hum Neurosci. 2024 Dec 9;18:1504783. doi: 10.3389/fnhum.2024.1504783. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39717149 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

