Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis
- PMID: 32913280
- PMCID: PMC7483525
- DOI: 10.1038/s41467-020-18113-4
Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis
Abstract
Traumatic brain injury (TBI) is a leading global cause of death and disability. Here we demonstrate in an experimental mouse model of TBI that mild forms of brain trauma cause severe deficits in meningeal lymphatic drainage that begin within hours and last out to at least one month post-injury. To investigate a mechanism underlying impaired lymphatic function in TBI, we examined how increased intracranial pressure (ICP) influences the meningeal lymphatics. We demonstrate that increased ICP can contribute to meningeal lymphatic dysfunction. Moreover, we show that pre-existing lymphatic dysfunction before TBI leads to increased neuroinflammation and negative cognitive outcomes. Finally, we report that rejuvenation of meningeal lymphatic drainage function in aged mice can ameliorate TBI-induced gliosis. These findings provide insights into both the causes and consequences of meningeal lymphatic dysfunction in TBI and suggest that therapeutics targeting the meningeal lymphatic system may offer strategies to treat TBI.
Conflict of interest statement
Competing interests: J.K. is an advisor to PureTech Health/Ariya. All other authors declare no competing interests.
Figures
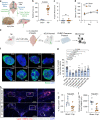

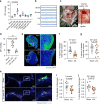
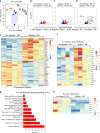
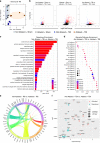


Comment in
-
Meningeal lymphatic flow slows after mild traumatic brain injury.Nat Rev Neurol. 2020 Nov;16(11):600. doi: 10.1038/s41582-020-00420-5. Nat Rev Neurol. 2020. PMID: 32973368 No abstract available.
-
Potential gene therapy for FTD.Nat Rev Neurol. 2020 Nov;16(11):600. doi: 10.1038/s41582-020-00423-2. Nat Rev Neurol. 2020. PMID: 33009521 No abstract available.
References
-
- The changing landscape of traumatic brain injury research. Lancet Neurol. 11, 651, 10.1016/S1474-4422(12)70166-7 (2012). - PubMed
-
- Mortimer JA, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J. Epidemiol. 1991;20(Suppl 2):S28–S35. - PubMed
-
- Holsinger T, et al. Head injury in early adulthood and the lifetime risk of depression. Arch. Gen. Psychiatry. 2002;59:17–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

