A systematic review of meta-analyses assessing the validity of tumour response endpoints as surrogates for progression-free or overall survival in cancer
- PMID: 32913287
- PMCID: PMC7687906
- DOI: 10.1038/s41416-020-01050-w
A systematic review of meta-analyses assessing the validity of tumour response endpoints as surrogates for progression-free or overall survival in cancer
Abstract
Background: Tumour response endpoints, such as overall response rate (ORR) and complete response (CR), are increasingly used in cancer trials. However, the validity of response-based surrogates is unclear. This systematic review summarises meta-analyses assessing the association between response-based outcomes and overall survival (OS), progression-free survival (PFS) or time-to-progression (TTP).
Methods: Five databases were searched to March 2019. Meta-analyses reporting correlation or regression between response-based outcomes and OS, PFS or TTP were summarised.
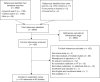
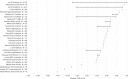
Results: The systematic review included 63 studies across 20 cancer types, most commonly non-small cell lung cancer (NSCLC), colorectal cancer (CRC) and breast cancer. The strength of association between ORR or CR and either PFS or OS varied widely between and within studies, with no clear pattern by cancer type. The association between ORR and OS appeared weaker and more variable than that between ORR and PFS, both for associations between absolute endpoints and associations between treatment effects.
Conclusions: This systematic review suggests that response-based endpoints, such as ORR and CR, may not be reliable surrogates for PFS or OS. Where it is necessary to use tumour response to predict treatment effects on survival outcomes, it is important to fully reflect all statistical uncertainty in the surrogate relationship.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Taylor RS, Elston J. The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports. Health Technol. Assess. 2009;13:1–72. - PubMed
-
- Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 1996;125:605–613. - PubMed
-
- Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 1989;8:431–440. - PubMed
-
- Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

