Fluid Biomarkers for Synaptic Dysfunction and Loss
- PMID: 32913390
- PMCID: PMC7444114
- DOI: 10.1177/1177271920950319
Fluid Biomarkers for Synaptic Dysfunction and Loss
Abstract
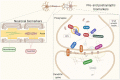
Synapses are the site for brain communication where information is transmitted between neurons and stored for memory formation. Synaptic degeneration is a global and early pathogenic event in neurodegenerative disorders with reduced levels of pre- and postsynaptic proteins being recognized as a core feature of Alzheimer's disease (AD) pathophysiology. Together with AD, other neurodegenerative and neurodevelopmental disorders show altered synaptic homeostasis as an important pathogenic event, and due to that, they are commonly referred to as synaptopathies. The exact mechanisms of synapse dysfunction in the different diseases are not well understood and their study would help understanding the pathogenic role of synaptic degeneration, as well as differences and commonalities among them and highlight candidate synaptic biomarkers for specific disorders. The assessment of synaptic proteins in cerebrospinal fluid (CSF), which can reflect synaptic dysfunction in patients with cognitive disorders, is a keen area of interest. Substantial research efforts are now directed toward the investigation of CSF synaptic pathology to improve the diagnosis of neurodegenerative disorders at an early stage as well as to monitor clinical progression. In this review, we will first summarize the pathological events that lead to synapse loss and then discuss the available data on established (eg, neurogranin, SNAP-25, synaptotagmin-1, GAP-43, and α-syn) and emerging (eg, synaptic vesicle glycoprotein 2A and neuronal pentraxins) CSF biomarkers for synapse dysfunction, while highlighting possible utilities, disease specificity, and technical challenges for their detection.
Keywords: Alzheimer’s disease; Synaptic biomarkers; cerebrospinal fluid; proteomics; synaptopathies.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Figures

References
-
- Frankish H, Horton R. Prevention and management of dementia: a priority for public health. Lancet (London, England). 2017;390:2614-2615. - PubMed
-
- Masliah E, Mallory M, Alford M, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56:127-129. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

