Reduction of Polyunsaturated Fatty Acids with Tumor Progression in a Lean Non-Alcoholic Steatohepatitis-Associated Hepatocellular Carcinoma Mouse Model
- PMID: 32913449
- PMCID: PMC7477439
- DOI: 10.7150/jca.48495
Reduction of Polyunsaturated Fatty Acids with Tumor Progression in a Lean Non-Alcoholic Steatohepatitis-Associated Hepatocellular Carcinoma Mouse Model
Abstract
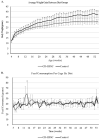
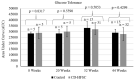
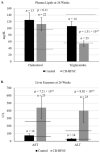
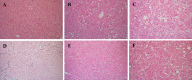
Background and Aim: Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in Western countries. While obesity and diabetes are the hallmarks of NAFLD, it also develops in lean individuals in the absence of metabolic syndrome, with a prevalence of 7 percent in the U.S. and 25-30 percent in some Asian countries. NAFLD represents the spectrum of liver disease, starting with excess liver fat accumulation (NAFL) that can progress to nonalcoholic steatohepatitis (NASH), cirrhosis and ultimately hepatocellular carcinoma (HCC). To date, the pathogenesis of lean NASH-HCC is poorly understood and a mouse model is lacking. We aimed to develop a mouse model of lean NASH-HCC using a choline deficient and high trans-fat/sucrose/cholesterol diet to enable better understanding of its molecular pathogenesis. Methods: C57BL/6N mice were fed this diet starting at 4 weeks of age for 52 weeks and were compared to mice fed an isocaloric low fat control diet for the same duration. C57BL/6N mice were chosen instead of the C57BL/6J mice due to the high susceptibility of C57BL/6J mice to diet-induced obesity. The plasma and tumor fatty acid profile of these mice was also investigated. Results: Nearly 61% of the mice developed lean NASH-HCC. These mice showed reduction of plasma polyunsaturated fatty acids (PUFAs) (linolenic acids (α and γ, ω-3 and ω-6, respectively), eicosapentanoic acid (ω-3), docosahexanoic acid (ω-3), and linoleic acid (ω-6)) and increasing levels over time in mice with pre-malignant lesions. Conclusions: We developed a novel high penetrance diet-induced lean NASH-HCC mouse model. Plasma PUFA levels were reduced with tumor progression in parallel with reduced expression of genes controlling desaturase expression suggesting their potential use as biomarkers for lean NASH-HCC progression as well as chemopreventive molecules.
Keywords: fat; liver cancer; non-alcoholic fatty liver disease; sugar; unhealthy diet.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Differential progression of unhealthy diet-induced hepatocellular carcinoma in obese and non-obese mice.PLoS One. 2022 Aug 22;17(8):e0272623. doi: 10.1371/journal.pone.0272623. eCollection 2022. PLoS One. 2022. PMID: 35994501 Free PMC article.
-
Differential methylation patterns in lean and obese non-alcoholic steatohepatitis-associated hepatocellular carcinoma.BMC Cancer. 2022 Dec 6;22(1):1276. doi: 10.1186/s12885-022-10389-7. BMC Cancer. 2022. PMID: 36474183 Free PMC article.
-
MicroRNA expression analysis in high fat diet-induced NAFLD-NASH-HCC progression: study on C57BL/6J mice.BMC Cancer. 2016 Jan 5;16:3. doi: 10.1186/s12885-015-2007-1. BMC Cancer. 2016. PMID: 26728044 Free PMC article.
-
Potential for dietary ω-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer.Adv Nutr. 2015 Nov 13;6(6):694-702. doi: 10.3945/an.115.009423. Print 2015 Nov. Adv Nutr. 2015. PMID: 26567194 Free PMC article. Review.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
Unveiling the potential anticancer activity of Spirulina maxima extract-nanoemulsion through in vitro and in vivo studies.Sci Rep. 2025 Jan 6;15(1):912. doi: 10.1038/s41598-024-82924-4. Sci Rep. 2025. PMID: 39762303 Free PMC article.
-
Epigenetic Regulation in Lean Nonalcoholic Fatty Liver Disease.Int J Mol Sci. 2023 Aug 16;24(16):12864. doi: 10.3390/ijms241612864. Int J Mol Sci. 2023. PMID: 37629043 Free PMC article. Review.
-
The Role of Fatty Acids in Non-Alcoholic Fatty Liver Disease Progression: An Update.Int J Mol Sci. 2021 Jun 27;22(13):6900. doi: 10.3390/ijms22136900. Int J Mol Sci. 2021. PMID: 34199035 Free PMC article. Review.
-
Lipid alterations in chronic liver disease and liver cancer.JHEP Rep. 2022 Mar 26;4(6):100479. doi: 10.1016/j.jhepr.2022.100479. eCollection 2022 Jun. JHEP Rep. 2022. PMID: 35469167 Free PMC article. Review.
-
Experimental diets dictate the metabolic benefits of probiotics in obesity.Gut Microbes. 2023 Jan-Dec;15(1):2192547. doi: 10.1080/19490976.2023.2192547. Gut Microbes. 2023. PMID: 36945120 Free PMC article.
References
-
- Suzuki A, Diehl AM. Nonalcoholic Steatohepatitis. Annu Rev Med. 2017;68:85–98. - PubMed
-
- Dyson J, Jaques B, Chattopadyhay D. et al. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60(1):110–117. - PubMed
-
- Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26(9):2183–2191. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

