Safety, Efficacy, Pharmacokinetic and Pharmacodynamic evaluation of YF-H-2015005 for mobilizing Hematopoietic stem cells in Non-Hodgkin's Lymphoma Patients
- PMID: 32913458
- PMCID: PMC7477452
- DOI: 10.7150/jca.48748
Safety, Efficacy, Pharmacokinetic and Pharmacodynamic evaluation of YF-H-2015005 for mobilizing Hematopoietic stem cells in Non-Hodgkin's Lymphoma Patients
Abstract
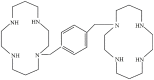
Background: Targeting the interaction between SDF1 and CXCR4 may provide an opportunity to intervene in the hematopoietic stem cell mobilization process. Aim: The present study aimed to investigate the safety, efficacy, pharmacokinetic and pharmacodynamic profiles of YF-H-2015005, a CXCR4 antagonist, for the mobilization of hematopoietic stem cells (HSCs). Methods: A total of 15 patients with non-Hodgkin's lymphoma (NHL) eligible for autologous hematopoietic stem cell transplantation were enrolled. All patients achieved a partial or complete remission after the first- or second-line therapy. Granulocyte colony stimulating factor (G-CSF) was given in the morning for 8 consecutive days, and 0.24 mg/kg YF-H-2015005 was subcutaneously administered in the evening of the 4th day of G-CSF treatment for up to four days. Apheresis was performed 9-10 hours following each dose of YF-H-2015005. Results: YF-H-2015005 was rapidly absorbed and eliminated, with Tmax and t1/2 of 0.5 and 5.04 ± 1.00 hours, respectively. Moreover, the mean peripheral blood CD34+ cell counts were elevated by 2.0- to 2.9-fold from 2 to 24 hours, and reached the maximum level of 76.5 ± 53.9 cells/kg at 10 hours after YF-H-2015005 treatment. Fourteen (93%) out of 15 NHL patients achieved a minimum target of ≥2×106/kg CD34+ cells. Furthermore, there was no grades 3-4 treatment-related adverse event observed among these patients. Conclusion: YF-H-2015005 can serve as a safe, effective agent in combination with G-CSF for CD34+ hematopoietic progenitor cell mobilization in NHL patients.
Keywords: Drug Evaluation; Hematopoietic Stem Cell Mobilization; Lymphoma, Non-Hodgkin's; Pharmacokinetics; Safety.
© The author(s).
Conflict of interest statement
Competing Interests: Shufang Wang is an employee of Hefei Yifan Biopharmaceuticals Inc. The other authors declare that they have no conflicts of interest.
Figures
Similar articles
-
YF-H-2015005, a CXCR4 Antagonist, for the Mobilization of Hematopoietic Stem Cells in Non-Hodgkin Lymphoma Patients: A Randomized, Controlled, Phase 3 Clinical Trial.Front Med (Lausanne). 2021 Feb 2;8:609116. doi: 10.3389/fmed.2021.609116. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33604348 Free PMC article.
-
Peripheral blood stem cell mobilization with cyclophosphamide in combination with G-CSF, GM-CSF, or sequential GM-CSF/G-CSF in non-Hodgkin's lymphoma patients: a randomized prospective study.J Hematother Stem Cell Res. 2000 Oct;9(5):737-48. doi: 10.1089/15258160050196786. J Hematother Stem Cell Res. 2000. PMID: 11091498 Clinical Trial.
-
High-dose therapy followed by autologous peripheral-blood stem-cell transplantation for patients with Hodgkin's disease and non-Hodgkin's lymphoma using unprimed and granulocyte colony-stimulating factor-mobilized peripheral-blood stem cells.J Clin Oncol. 1994 Oct;12(10):2176-86. doi: 10.1200/JCO.1994.12.10.2176. J Clin Oncol. 1994. PMID: 7523609 Clinical Trial.
-
Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with recurrent indolent non-Hodgkin's lymphoma.Biol Blood Marrow Transplant. 2001;7(12):680-7. doi: 10.1053/bbmt.2001.v7.pm11787531. Biol Blood Marrow Transplant. 2001. PMID: 11787531 Clinical Trial.
-
Mobilization of peripheral-blood progenitor cells with high-dose etoposide and granulocyte colony-stimulating factor in patients with breast cancer, non-Hodgkin's lymphoma, and Hodgkin's disease.J Clin Oncol. 1997 Feb;15(2):759-65. doi: 10.1200/JCO.1997.15.2.759. J Clin Oncol. 1997. PMID: 9053502
Cited by
-
YF-H-2015005, a CXCR4 Antagonist, for the Mobilization of Hematopoietic Stem Cells in Non-Hodgkin Lymphoma Patients: A Randomized, Controlled, Phase 3 Clinical Trial.Front Med (Lausanne). 2021 Feb 2;8:609116. doi: 10.3389/fmed.2021.609116. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33604348 Free PMC article.
References
-
- Müller TA, Pennisi S, Zwick A. et al. PIM1 inhibition effectively enhances plerixafor-induced HSC mobilization by counteracting CXCR4 upregulation and blocking CXCL12 secretion. Leukemia. 2019;33:1296–1301. - PubMed
-
- Sugiyama T, Omatsu Y, Nagasawa T. Niches for hematopoietic stem cells and immune cell progenitors. Int Immunol. 2019;31:5–11. - PubMed
LinkOut - more resources
Full Text Sources