Role for contrast-enhanced ultrasound in assessing complications after kidney transplant
- PMID: 32913562
- PMCID: PMC7457161
- DOI: 10.4329/wjr.v12.i8.156
Role for contrast-enhanced ultrasound in assessing complications after kidney transplant
Abstract
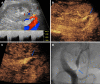
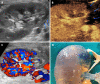
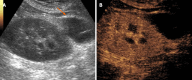
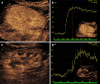
Kidney transplantation (KT) is an effective treatment for end-stage renal disease. Despite their rate has reduced over time, post-transplant complications still represent a major clinical problem because of the associated risk of graft failure and loss. Thus, post-KT complications should be diagnosed and treated promptly. Imaging plays a pivotal role in this setting. Grayscale ultrasound (US) with color Doppler analysis is the first-line imaging modality for assessing complications, although many findings lack specificity. When performed by experienced operators, contrast-enhanced US (CEUS) has been advocated as a safe and fast tool to improve the accuracy of US. Also, when performing CEUS there is potentially no need for further imaging, such as contrast-enhanced computed tomography or magnetic resonance imaging, which are often contraindicated in recipients with impaired renal function. This technique is also portable to patients' bedside, thus having the potential of maximizing the cost-effectiveness of the whole diagnostic process. Finally, the use of blood-pool contrast agents allows translating information on graft microvasculature into time-intensity curves, and in turn quantitative perfusion indexes. Quantitative analysis is under evaluation as a tool to diagnose rejection or other causes of graft dysfunction. In this paper, we review and illustrate the indications to CEUS in the post-KT setting, as well as the main CEUS findings that can help establishing the diagnosis and planning the most adequate treatment.
Keywords: Contrast-enhanced ultrasound; Graft function; Kidney transplant; Post-renal transplant complications; Ultrasound contrast agents.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Figures



References
-
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11:2093–2109. - PubMed
-
- Haberal M, Boyvat F, Akdur A, Kırnap M, Özçelik Ü, Yarbuğ Karakayalı F. Surgical Complications After Kidney Transplantation. Exp Clin Transplant. 2016;14:587–595. - PubMed
-
- Expert Panel on Urologic Imaging, Taffel MT, Nikolaidis P, Beland MD, Blaufox MD, Dogra VS, Goldfarb S, Gore JL, Harvin HJ, Heilbrun ME, Heller MT, Khatri G, Preminger GM, Purysko AS, Smith AD, Wang ZJ, Weinfeld RM, Wong-You-Cheong JJ, Remer EM, Lockhart ME. ACR Appropriateness Criteria® Renal Transplant Dysfunction. J Am Coll Radiol. 2017;14:S272–S281. - PubMed
-
- Sugi MD, Joshi G, Maddu KK, Dahiya N, Menias CO. Imaging of Renal Transplant Complications throughout the Life of the Allograft: Comprehensive Multimodality Review. Radiographics. 2019;39:1327–1355. - PubMed
-
- Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F, Oyen R, Kuypers D, Evenepoel P, Bammens B, Sprangers B, Meijers B, Pirenne J, Monbaliu D, de Jonge H, Metalidis C, De Vusser K, Vanrenterghem Y. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369:1797–1806. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

