Sonographic evaluation of prostatic artery embolization: Far beyond size measurements
- PMID: 32913563
- PMCID: PMC7457160
- DOI: 10.4329/wjr.v12.i8.172
Sonographic evaluation of prostatic artery embolization: Far beyond size measurements
Abstract
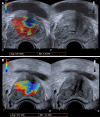
Prostatic artery embolization (PAE) has gained acceptance as a minimally invasive, safe and effective treatment of symptomatic benign prostatic hyperplasia. Radiologic imaging is an indispensable part of post-interventional evaluation of PAE and serves both clinical and investigational purposes. In this context, ultrasonography (US) has a central and multifaceted role. Gray-scale US is routinely utilized for measurement of significant outcome parameters (prostatic volume, intra-vesical prostatic protrusion and post-void residual volume) before and after PAE. Improvement of these parameters may become more obvious one-month post-PAE, or later. Contrast-enhanced US (CEUS) with intravenous administration of a second-generation echo-enhancer can demonstrate prostatic infarcts (as enhancement defects) immediately post-PAE and monitor their resolution over time. The volume of prostatic infarcts can also be measured and compared to prostatic volume. Prostatic infarction is a definite sign of the local efficacy of PAE and a predictor of prostate shrinkage and (at least in some patients) of clinical success. CEUS can also be performed intraoperatively in the angio-suite, for on-site evaluation of the ischemic effect; a variation of this technique, with intraarterial (instead of intravenous) administration of diluted echo enhancer, can also be applied intraoperatively, to map the embolized territory and to prevent non-target embolization. Initial experience with US-elastographic techniques (shear-wave and strain elastography) has shown that they can detect and quantify the improvement of tissue elasticity post-PAE, thus providing new insights into the therapeutic mechanisms of this treatment. With utilization of high-end equipment, experience and standardized imaging protocols, US could be the primary modality for imaging evaluation of PAE.
Keywords: Benign prostatic hyperplasia; Contrast-enhanced ultrasound; Infarction; Prostatic artery embolization; Shear-wave elastography; Strain elastography; Ultrasound.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There is no conflict of interest associated with any of the authors of this manuscript.
Figures





References
-
- Kuang M, Vu A, Athreya S. A Systematic Review of Prostatic Artery Embolization in the Treatment of Symptomatic Benign Prostatic Hyperplasia. Cardiovasc Intervent Radiol. 2017;40:655–663. - PubMed
-
- Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clin Radiol. 2017;72:16–22. - PubMed
-
- Teichgräber U, Aschenbach R, Diamantis I, von Rundstedt FC, Grimm MO, Franiel T. Prostate Artery Embolization: Indication, Technique and Clinical Results. Rofo. 2018;190:847–855. - PubMed
-
- Sun F, Crisóstomo V, Báez-Díaz C, Sánchez FM. Prostatic Artery Embolization (PAE) for Symptomatic Benign Prostatic Hyperplasia (BPH): Part 2, Insights into the Technical Rationale. Cardiovasc Intervent Radiol. 2016;39:161–169. - PubMed
-
- Fernandes L, Rio Tinto H, Pereira J, Duarte M, Bilhim T, Martins Pisco J. Prostatic arterial embolization: post-procedural follow-up. Tech Vasc Interv Radiol. 2012;15:294–299. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

