Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers
- PMID: 32913623
- PMCID: PMC7444096
- DOI: 10.1177/2041731420947242
Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers
Abstract
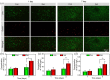
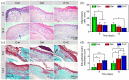
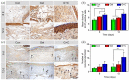
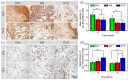
A diabetic foot ulcer (DFUs) is a state of prolonged chronic inflammation, which can result in amputation. Different from normal skin wounds, various commercially available dressings have not sufficiently improved the healing of DFUs. In this study, a novel self-healing hydrogel was prepared by in situ crosslinking of N-carboxyethyl chitosan (N-chitosan) and adipic acid dihydrazide (ADH) with hyaluronic acid-aldehyde (HA-ALD), to provide a moist and inflammatory relief environment to promote stem cell proliferation or secretion of growth factors, thus accelerating wound healing. The results demonstrated that this injectable and self-healing hydrogel has excellent swelling properties, stability, and mechanical properties. This biocompatible hydrogel stimulated secretion of growth factors from bone marrow mesenchymal stem cells (BM-MSCs) and regulated the inflammatory environment by inhibiting the expression of M1 macrophages and promoting the expression of M2 macrophages, resulting in granulation tissue formation, collagen deposition, nucleated cell proliferation, neovascularization, and enhanced diabetic wound healing. This study showed that N-chitosan/HA-ALD hydrogel could be used as a multifunctional injectable wound dressing to regulate chronic inflammation and provide an optimal environment for BM-MSCs to promote diabetic wound healing.
Keywords: BM-MSCs; Hydrogel; diabetic wound; inflammatory micro-environment.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Braffett BH, Gubitosi-Klug RA, Albers JW. Risk factors for diabetic peripheral neuropathy and cardiovascular autonomic neuropathy in the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study. Diabetes 2020; 69(5): 1000–1010. - PMC - PubMed
-
- Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94(3): 311–321. - PubMed
-
- Moura LIF, Dias AMA, Carvalho E, et al. Recent advances on the development of wound dressings for diabetic foot ulcer treatment-A review. Acta Biomater 2013; 9(7): 7093–7114. - PubMed
-
- Zhao Y, Li Z, Song S, et al. Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv Funct Mater 2019; 29(31): 1901474.
LinkOut - more resources
Full Text Sources
Other Literature Sources

