Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis
- PMID: 32913710
- PMCID: PMC7452318
- DOI: 10.1016/j.jot.2020.03.015
Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis
Abstract
Stem cells are considered to be one of the greatest potential treatments to cure degenerative diseases. Stem cells injection for knee osteoarthritis (OA) is still a relatively new treatment and has not yet gained popularity. So, the effectiveness, safety and potential of mesenchymal stem cells (MSCs) for knee OA treatment is worthy to be explored. Explore the effectiveness and safety of mesenchymal stem cells (MSCs) in the treatment of knee osteoarthritis. We collected clinical trials using MSCs as treatment for knee OA (before April 2019), including randomized controlled trials (RCTs), retrospective studies and cohort studies. We searched PubMed, EMBASE, Cochrane Library, Web of Science and the ClinicalTrials.gov with keywords (Mesenchymal stem cells [MSCs], Knee osteoarthritis, Effectiveness and Safety), and then performed a systematic review and cumulative metaanalysis of all RCTs and retrospective comparative studies. To evaluate the effectiveness and safety of MSC in knee OA treatment, we applied visual analog scale score, Western Ontario and McMaster Universities Osteo-arthritis Index and adverse events. We included 15 RCTs, two retrospective studies and two cohort studies including a total of 584 knee OA patients in this study. We demonstrated that MSC treatment could significantly decrease visual analog scale in a 12-month follow-up study compared with controls (p < 0.001). MSC therapy also showed significant decreases in Western Ontario and McMaster Universities Osteoarthritis Index scores after the 6-month follow-up (p < 0.001). MSC therapy showed no difference compared with controls (p > 0.05) in adverse events. We suggest that MSC therapy could serve as an effective and safe therapy for clinical application in OA treatment.
The translational potential of this article: This study provided the best available evidence and a wider perspective to MSCs application in the management of knee OA. MSCs therapy will have great translational potential in the clinical treatment of various degenerative diseases once optimum formula and explicit target population are identified.
Keywords: Knee osteoarthritis; Mesenchymal stem cells; Meta-analysis.
© 2020 Published by Elsevier (Singapore) Pte Ltd on behalf of Chinese Speaking Orthopaedic Society.
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to this article.
Figures
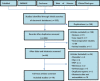
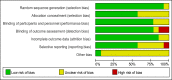





References
-
- Koh Y.G., Choi Y.J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. The Knee. 2012;19(6):902–907. - PubMed
-
- Ghosh P., Smith M. Osteoarthritis, genetic and molecular mechanisms. Biogerontology. 2002;3(1–2):85–88. - PubMed
-
- Vega Aurelio, Martín-Ferrero Miguel Angel, Canto Francisco Del, Alberca Mercedes, García Veronica, Munar Anna. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells. Transplantation. 2015;99(8):1681–1690. - PubMed
-
- Wang Y.L., Jin W.X., Liu H.Y., Cui Y.H., Mao Q.C., Fei Z.Y. Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis. Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery. 2016;30(12):1472. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

