Real-World Utility of an Amplicon-Based Next-Generation Sequencing Liquid Biopsy for Broad Molecular Profiling in Patients With Advanced Non-Small-Cell Lung Cancer
- PMID: 32914037
- PMCID: PMC7446523
- DOI: 10.1200/PO.18.00211
Real-World Utility of an Amplicon-Based Next-Generation Sequencing Liquid Biopsy for Broad Molecular Profiling in Patients With Advanced Non-Small-Cell Lung Cancer
Abstract
Purpose: To assess the feasibility and utility of circulating tumor DNA (ctDNA) by amplicon-based next-generation sequencing (NGS) analysis in the daily clinical setting in a cohort of patients with advanced non-small-cell lung cancer (NSCLC), as an alternative approach to tissue molecular profiling.
Patients and methods: In this single-center prospective study, treatment-naïve and previously treated patients with advanced NSCLC were enrolled. Clinical validation of ctDNA using amplicon-based NGS analysis (with a 36-gene panel) was performed against standard-of-care tissue molecular analysis in treatment-naïve patients. The feasibility, utility, and prognostic value of ctDNA as a dynamic marker of treatment efficacy was evaluated. Results of tissue molecular profile were blinded during ctDNA analysis.
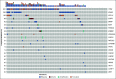
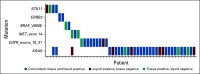
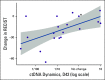
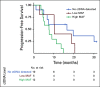
Results: Of 214 patients with advanced NSCLC who were recruited, 156 were treatment-naïve patients and 58 were pretreated patients with unknown tissue molecular profile. ctDNA screening was successfully performed for 91% (n = 194) of all patients, and mutations were detected in 77% of these patients. Tissue molecular analysis was available for 111 patients (52%), and tissue somatic mutations were found for 78% (n = 87) of patients. For clinically relevant variants, concordance agreement between ctDNA and tumor tissue analysis was 95% among 94 treatment-naïve patients who had concurrent liquid and tumor biopsy molecular profiles. Sensitivity and specificity were 81% and 97%, respectively. Of the 103 patients with no tissue available, ctDNA detected potential actionable mutations in 17% of patients; of these, 10% received personalized treatment. ctDNA kinetics correlated with response rate and progression-free survival in 31 patients treated with first-line platinum-based chemotherapy.
Conclusion: These real-world data from a prospective study endorse ctDNA molecular profile by amplicon-based NGS as an accurate and reliable tool to detect and monitor clinically relevant molecular alterations in patients with advanced NSCLC.
© 2019 by American Society of Clinical Oncology.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org or ascopubs.org/po/author-center.Jordi RemonConsulting or Advisory Role: Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, MSD Oncology Travel, Accommodations, Expenses: Roche, Genentech, Inivata, OSE ImmunotherapeuticsCaroline CaramellaConsulting or Advisory Role: Bristol-Myers Squibb, PfizerKaren HowarthEmployment: Inivata Stock and Other Ownership Interests: Inivata Research Funding: Inivata Patents, Royalties, Other Intellectual Property: Patents and patent applications relating to cancer classifications, detection or analysis of microRNA and circulating tumor DNA, detection of rare sequence variants, applications in molecular diagnosticsVincent PlagnolEmployment: Inivata Stock and Other Ownership Interests: Inivata Patents, Royalties, Other Intellectual Property: Inivata patentsNitzan RosenfeldEmployment: Storm Therapeutics (I), Inivata Leadership: Inivata Stock and Other Ownership Interests: Inivata, Mission Therapeutics (I) Research Funding: AstraZeneca (Inst) Patents, Royalties, Other Intellectual Property: Patents and patent applications relating to cancer classifications, detection or analysis of microRNA and circulating tumor DNA, detection of rare sequence variants, applications in molecular diagnosticsClive MorrisEmployment: Inivata Leadership: Inivata Stock and Other Ownership Interests: InivataCecile Le PechouxConsulting or Advisory Role: AstraZeneca, Lilly, Nanobiotix, AmgenJulien AdamConsulting or Advisory Role: Roche, Bristol-Myers Squibb, AstraZeneca, Merck Sharp & Dohme Research Funding: Sanofi (Inst), Pierre Fabre (Inst), Merck Sharp & Dohme (Inst)David PlanchardConsulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Novartis, Roche, Pfizer, MSD Oncology, CelgeneGilles VassalConsulting or Advisory Role: Bayer, Roche, Genentech, AstraZeneca, Bristol-Myers Squibb, Celgene, Lilly, Servier, Takeda, Incyte, Ipsen, Novartis, Merck Serono Travel, Accommodations, Expenses: Bristol-Myers Squibb, RocheEmma GreenEmployment: Inivata Stock and Other Ownership Interests: Inivata Travel, Accommodations, Expenses: InivataJean-Charles SoriaEmployment: MedImmune Stock and Other Ownership Interests: AstraZeneca, Gritstone Oncology Honoraria: Roche, AstraZeneca, Sanofi, Servier, Pierre Fabre, Abbvie, Pharmamar-ZeltiaBenjamin BesseResearch Funding: AstraZeneca (Inst), Roche (Inst), Genentech (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Servier (Inst), Onxeo (Inst), Bristol-Myers Squibb (Inst), Ose Pharma (Inst), Inivata (Inst), Novartis (Ins), OncoMed (Inst), Loxo (Inst) Travel, Accommodations, Expenses: Roche, Pfizer, Bristol-Myers Squibb, Medarex, Novartis, Pierre Fabre No other potential conflicts of interest were reported.
Figures




References
-
- Reck M, Rabe KF. Precision diagnosis and treatment for advanced non–small-cell lung cancer. N Engl J Med. 2017;377:849–861. - PubMed
-
- Barlesi F, Mazieres J, Merlio J-P, et al. Routine molecular profiling of patients with advanced non–small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387:1415–1426. - PubMed
-
- Novello S, Barlesi F, Califano R, et al. Metastatic non–small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1–v27. - PubMed
-
- Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol. 2018;36:911–919. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

