Pickering emulsions stabilized by colloidal gel particles complexed or conjugated with biopolymers to enhance bioaccessibility and cellular uptake of curcumin
- PMID: 32914133
- PMCID: PMC7473359
- DOI: 10.1016/j.crfs.2020.05.001
Pickering emulsions stabilized by colloidal gel particles complexed or conjugated with biopolymers to enhance bioaccessibility and cellular uptake of curcumin
Abstract
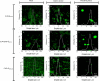
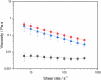
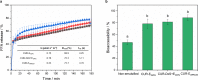
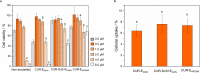
The aim of this study was to investigate the fate of curcumin (CUR)-loaded Pickering emulsions with complex interfaces during in vitro gastrointestinal transit and test the efficacy of such emulsions on improving the bioaccessibility and cellular uptake of CUR. CUR-loaded Pickering emulsions tested were whey protein nanogel particle-stabilized Pickering emulsions (CUR-EWPN) and emulsions displaying complex interfaces included 1) layer-by-layer dextran sulphate-coated nanogel-stabilized Pickering emulsions (CUR-DxS+EWPN) and 2) protein+dextran-conjugated microgel-stabilized Pickering emulsions (CUR-EWPDxM). The hypothesis was that the presence of complex interfacial material at the droplet surface would provide better protection to the droplets against physiological degradation, particularly under gastric conditions and thus, improve the delivery of CUR to Caco-2 intestinal cells. The emulsions were characterized using droplet sizing, apparent viscosity, confocal and cryo-scanning electron microscopy, zeta-potential, lipid digestion kinetics, bioaccessibility of CUR as well as cell viability and uptake by Caco-2 cells. Emulsion droplets with modified to complex interfacial composition (i.e. CUR-DxS+EWPN and CUR-EWPDxM) provided enhanced kinetic stability to the Pickering emulsion droplets against coalescence in the gastric regime as compared to droplets having unmodified interface (i.e. CUR-EWPN), whereas droplet coalescence occurred in intestinal conditions irrespective of the initial interfacial materials. A similar rate and extent of free fatty acid release occurred in all the emulsions during intestinal digestion (p > 0.05), which correlated with the bioaccessibility of CUR. Striking, CUR-DxS+EWPN and CUR-EWPDxM significantly improved cellular CUR uptake as compared to CUR-EWPN (p < 0.05). These results highlight a promising new strategy of designing gastric-stable Pickering emulsions with complex interfaces to improve the delivery of lipophilic bioactive compounds to the cells for the future design of functional foods.
Keywords: Caco-2 cells; Cellular uptake; Curcumin; Microgel; Pickering emulsion; in vitro digestion.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interests.
Figures



References
-
- Amani S., Mohamadnia Z., Mahdavi A. pH-responsive hybrid magnetic polyelectrolyte complex based on alginate/BSA as efficient nanocarrier for curcumin encapsulation and delivery. Int. J. Biol. Macromol. 2019;141:1258–1270. - PubMed
-
- Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4(6):807–818. - PubMed
-
- Araiza-Calahorra A., Sarkar A. Pickering emulsion stabilized by protein nanogel particles for delivery of curcumin: effects of pH and ionic strength on curcumin retention. Food Struct. 2019;21:100113.
-
- Araiza-Calahorra A., Sarkar A. Designing biopolymer-coated Pickering emulsions to modulate in vitro gastric digestion: a static model study. Food Funct. 2019;10(9):5498–5509. - PubMed
-
- Araiza-Calahorra A., Akhtar M., Sarkar A. Recent advances in emulsion-based delivery approaches for curcumin: from encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018;71:155–169.
LinkOut - more resources
Full Text Sources

