Photochemical Methods for Peptide Macrocyclisation
- PMID: 32914455
- PMCID: PMC7821122
- DOI: 10.1002/chem.202003779
Photochemical Methods for Peptide Macrocyclisation
Abstract
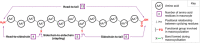
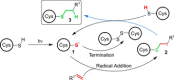
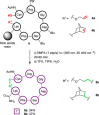
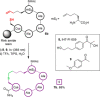
Photochemical reactions have been the subject of renewed interest over the last two decades, leading to the development of many new, diverse and powerful chemical transformations. More recently, these developments have been expanded to enable the photochemical macrocyclisation of peptides and small proteins. These constructs benefit from increased stability, structural rigidity and biological potency over their linear counterparts, providing opportunities for improved therapeutic agents. In this review, an overview of both the established and emerging methods for photochemical peptide macrocyclisation is presented, highlighting both the limitations and opportunities for further innovation in the field.
Keywords: macrocyclisation; peptides; photochemistry.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



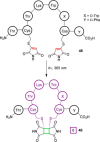
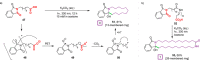
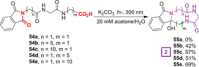
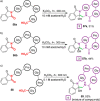


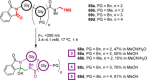


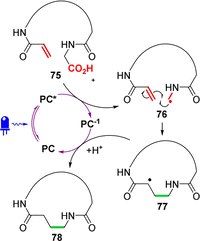
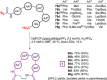
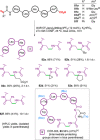
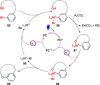
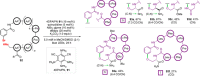
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

