Parasitoids indicate major climate-induced shifts in arctic communities
- PMID: 32914511
- PMCID: PMC7692897
- DOI: 10.1111/gcb.15297
Parasitoids indicate major climate-induced shifts in arctic communities
Abstract
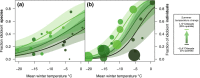
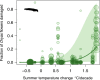
Climatic impacts are especially pronounced in the Arctic, which as a region is warming twice as fast as the rest of the globe. Here, we investigate how mean climatic conditions and rates of climatic change impact parasitoid insect communities in 16 localities across the Arctic. We focus on parasitoids in a widespread habitat, Dryas heathlands, and describe parasitoid community composition in terms of larval host use (i.e., parasitoid use of herbivorous Lepidoptera vs. pollinating Diptera) and functional groups differing in their closeness of host associations (koinobionts vs. idiobionts). Of the latter, we expect idiobionts-as being less fine-tuned to host development-to be generally less tolerant to cold temperatures, since they are confined to attacking hosts pupating and overwintering in relatively exposed locations. To further test our findings, we assess whether similar climatic variables are associated with host abundances in a 22 year time series from Northeast Greenland. We find sites which have experienced a temperature rise in summer while retaining cold winters to be dominated by parasitoids of Lepidoptera, with the reverse being true for the parasitoids of Diptera. The rate of summer temperature rise is further associated with higher levels of herbivory, suggesting higher availability of lepidopteran hosts and changes in ecosystem functioning. We also detect a matching signal over time, as higher summer temperatures, coupled with cold early winter soils, are related to high herbivory by lepidopteran larvae, and to declines in the abundance of dipteran pollinators. Collectively, our results suggest that in parts of the warming Arctic, Dryas is being simultaneously exposed to increased herbivory and reduced pollination. Our findings point to potential drastic and rapid consequences of climate change on multitrophic-level community structure and on ecosystem functioning and highlight the value of collaborative, systematic sampling effort.
Keywords: Dryas; Arctic; DNA barcoding; climate change; food webs; functional traits; host-parasitoid interactions; insect herbivory; pollinators.
© 2020 The Authors. Global Change Biology published by John Wiley & Sons Ltd.
Figures




References
-
- Ahola, M. , & Silvonen, K. (2005). Larvae of Northern European Noctuidae. Vaasa, Finland: Kuvaseppälä Yhtiöt Oy.
-
- Ammunét, T. , Kaukoranta, T. , Saikkonen, K. , Repo, T. , & Klemola, T. (2012). Invading and resident defoliators in a changing climate: Cold tolerance and predictions concerning extreme winter cold as a range‐limiting factor. Ecological Entomology, 37(3), 212–220. 10.1111/j.1365-2311.2012.01358.x - DOI
-
- Askew, R. R. , & Shaw, M. R. (1986). Parasitoid communities: Their size, structure and development In Waage J. & Greathead D. (Eds.), Insect parasitoids (pp. 225–264). London, UK: Academic Press.
MeSH terms
Grants and funding
- Parks Canada
- University of Guelph
- Societas pro Fauna et Flora Fennica
- 201500090/Maj ja Tor Nesslingin Säätiö
- 201600034/Maj ja Tor Nesslingin Säätiö
- 201700420/Maj ja Tor Nesslingin Säätiö
- Polar Knowledge Canada
- 152468-051/Icelandic Centre for Research
- Fonds Québécois de la Recherche sur la Nature et les Technologies
- The Danish Environmental Protection Agency
- Churchill Northern Studies Centre
- Entomological Society of Canada
- Canadian Polar Commission
- Polar Continental Shelf Project
- 276671/Biotieteiden ja Ympäristön Tutkimuksen Toimikunta
- 276909/Biotieteiden ja Ympäristön Tutkimuksen Toimikunta
- 285803/Biotieteiden ja Ympäristön Tutkimuksen Toimikunta
- Natural Sciences and Engineering Research Council of Canada
- 276909/Academy of Finland
- 285803/Academy of Finland
- 276671/Academy of Finland
- 201700420/Nessling Foundation
- 201600034/Nessling Foundation
- 201500090/Nessling Foundation
- Jane and Aatos Erkko Foundation
- French Polar Institute
- INTERACT
- 249902/F20/Research Council of Norway
- ArcticNet
- 18-05-60261/Russian Foundation for Basic Research
LinkOut - more resources
Full Text Sources

