Clinical Conditions "Suggestive of Progressive Supranuclear Palsy"-Diagnostic Performance
- PMID: 32914550
- PMCID: PMC7953080
- DOI: 10.1002/mds.28263
Clinical Conditions "Suggestive of Progressive Supranuclear Palsy"-Diagnostic Performance
Abstract
Background: The Movement Disorder Society diagnostic criteria for progressive supranuclear palsy introduced the diagnostic certainty level "suggestive of progressive supranuclear palsy" for clinical conditions with subtle signs, suggestive of the disease. This category aims at the early identification of patients, in whom the diagnosis may be confirmed as the disease evolves.
Objective: To assess the diagnostic performance of the defined clinical conditions suggestive of progressive supranuclear palsy in an autopsy-confirmed cohort.
Methods: Diagnostic performance of the criteria was analyzed based on retrospective clinical data of 204 autopsy-confirmed patients with progressive supranuclear palsy and 216 patients with other neurological diseases.
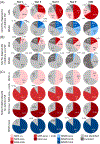
Results: The conditions suggestive of progressive supranuclear palsy strongly increased the sensitivity compared to the National Institute of Neurological Disorders and Stroke and Society for Progressive Supranuclear Palsy criteria. Within the first year after symptom onset, 40% of patients with definite progressive supranuclear palsy fulfilled criteria for suggestive of progressive supranuclear palsy. Two-thirds of patients suggestive of progressive supranuclear palsy evolved into probable progressive supranuclear palsy after an average of 3.6 years. Application of the criteria for suggestive of progressive supranuclear palsy reduced the average time to diagnosis from 3.8 to 2.2 years.
Conclusions: Clinical conditions suggestive of progressive supranuclear palsy allow earlier identification of patients likely to evolve into clinically possible or probable progressive supranuclear and to have underlying progressive supranuclear palsy pathology. Further work needs to establish the specificity and positive predictive value of this category in real-life clinical settings, and to develop specific biomarkers that enhance their diagnostic accuracy in early disease stages. © 2020 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: autopsy; clinical diagnostic criteria; early diagnosis; neuropathology; progressive supranuclear palsy; suggestive.
© 2020 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Conflict of interest statement
Figures



References
-
- Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 1996;55:97–105. - PubMed
-
- Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. J Neurol 1999;246: 6–15. - PubMed
-
- Rosler TW, Tayaranian Marvian A, Brendel M, et al. Four-repeat tauopathies. Prog Neurobiol 2019;180:101644. - PubMed
-
- Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

