ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura
- PMID: 32914582
- PMCID: PMC8146131
- DOI: 10.1111/jth.15006
ISTH guidelines for the diagnosis of thrombotic thrombocytopenic purpura
Erratum in
-
Erratum.J Thromb Haemost. 2021 May;19(5):1381. doi: 10.1111/jth.15304. J Thromb Haemost. 2021. PMID: 33880873 No abstract available.
Abstract
Background: Despite an increase in our understandings of pathogenesis of thrombotic thrombocytopenic purpura (TTP), the approaches for initial diagnosis and management of TTP vary significantly.
Objective: The evidence-based guidelines of the International Society on Thrombosis and Haemostasis (ISTH) are intended to support patients, clinicians, and other health care professionals in their decisions about the initial diagnosis and management of acute TTP.
Methods: In June 2018, ISTH formed a multidisciplinary panel that included hematologists, an intensive care physician, nephrologist, clinical pathologist, biostatistician, and patient representatives, as well as a methodology team from McMaster University. The panel composition was designed to minimize the potential conflicts of interests. The panel used the Grading of Recommendations Assessment, Development, and Evaluation approach and the Population, Intervention, Comparison, Outcome framework to develop and grade their recommendations. Public comments were sought and incorporated in the final document.
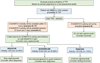
Results: The panel agreed on three recommendations covering the initial diagnosis with emphasis on the importance of ADAMTS13 testing (eg, activity, anti-ADAMTS13 IgG or inhibitor) and assessment of the pretest probability of TTP by clinical assessment and/or the risk assessment models like the PLASMIC or French score. The panel noted how availability and turnaround time of ADAMTS13 test results might affect early diagnosis and management, in particular the use of caplacizumab.
Conclusions: There is a lack of high-quality evidence to support strong recommendations for the initial diagnosis and management of a suspected TTP. The panel emphasized the importance of obtaining ADAMTS13 testing in a proper clinical context. Future research should focus on how to monitor and act on ADAMTS13 levels during remission.
Keywords: ADAMTS13; TTP; diagnosis; guidelines; thrombosis.
© 2020 International Society on Thrombosis and Haemostasis.
Figures

References
-
- Reese JA, Muthurajah DS, Kremer Hovinga JA, Vesely SK, Terrell DR, George JN. Children and adults with thrombotic thrombocytopenic purpura associated with severe, acquired Adamts13 deficiency: comparison of incidence, demographic and clinical features. Pediatr Blood Cancer. 2013;60:1676–1682. - PubMed
-
- Terrell DR, Williams LA, Vesely SK, Lammle B, Hovinga JA, George JN. The incidence of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: all patients, idiopathic patients, and patients with severe ADAMTS-13 deficiency. J Thromb Haemost. 2005;3:1432–1436. - PubMed
-
- Mariotte E, Azoulay E, Galicier L, et al. Epidemiology and pathophysiology of adulthood-onset thrombotic microangiopathy with severe ADAMTS13 deficiency (thrombotic thrombocytopenic purpura): a cross-sectional analysis of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3:e237–e245. - PubMed
-
- Levy GG, Nichols WC, Lian EC, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

