Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection
- PMID: 32914699
- PMCID: PMC7488171
- DOI: 10.1177/1073858420956905
Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection
Abstract
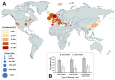
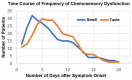
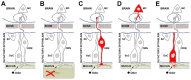
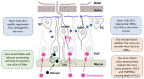
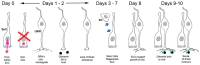
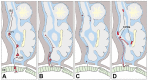
In recent months it has emerged that the novel coronavirus-responsible for the COVID-19 pandemic-causes reduction of smell and taste in a large fraction of patients. The chemosensory deficits are often the earliest, and sometimes the only signs in otherwise asymptomatic carriers of the SARS-CoV-2 virus. The reasons for the surprisingly early and specific chemosensory dysfunction in COVID-19 are now beginning to be elucidated. In this hypothesis review, we discuss implications of the recent finding that the prevalence of smell and taste dysfunction in COVID-19 patients differs between populations, possibly because of differences in the spike protein of different virus strains or because of differences in the host proteins that enable virus entry, thus modifying infectivity. We review recent progress in defining underlying cellular and molecular mechanisms of the virus-induced anosmia, with a focus on the emerging crucial role of sustentacular cells in the olfactory epithelium. We critically examine the current evidence whether and how the SARS-CoV-2 virus can follow a route from the olfactory epithelium in the nose to the brain to achieve brain infection, and we discuss the prospects for using the smell and taste dysfunctions seen in COVID-19 as an early and rapid diagnostic screening tool.
Keywords: ACE2; COVID-19; SARS-CoV-2; anosmia; brain infection; diagnosis; hyposmia; olfactory epithelium; prevalence; smell loss; taste.
Conflict of interest statement
Figures
Similar articles
-
Anosmia in COVID-19: A Bumpy Road to Establishing a Cellular Mechanism.ACS Chem Neurosci. 2020 Aug 5;11(15):2152-2155. doi: 10.1021/acschemneuro.0c00406. Epub 2020 Jul 16. ACS Chem Neurosci. 2020. PMID: 32673476 Free PMC article. Review.
-
COVID-19, cilia, and smell.FEBS J. 2020 Sep;287(17):3672-3676. doi: 10.1111/febs.15491. Epub 2020 Aug 6. FEBS J. 2020. PMID: 32692465 Free PMC article. Review.
-
Olfactory function and viral recovery in COVID-19.Brain Behav. 2021 Mar;11(3):e02006. doi: 10.1002/brb3.2006. Epub 2021 Jan 19. Brain Behav. 2021. PMID: 33465295 Free PMC article.
-
Reversible Taste Loss in a COVID-19 Patient With Preexisting Chronic Smell Impairment.J Investig Med High Impact Case Rep. 2021 Jan-Dec;9:2324709621990765. doi: 10.1177/2324709621990765. J Investig Med High Impact Case Rep. 2021. PMID: 33535814 Free PMC article.
-
COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters.Sci Transl Med. 2021 Jun 2;13(596):eabf8396. doi: 10.1126/scitranslmed.abf8396. Epub 2021 May 3. Sci Transl Med. 2021. PMID: 33941622 Free PMC article.
Cited by
-
A Comprehensive Review of COVID-19-Related Olfactory Deficiency: Unraveling Associations with Neurocognitive Disorders and Magnetic Resonance Imaging Findings.Diagnostics (Basel). 2024 Feb 7;14(4):359. doi: 10.3390/diagnostics14040359. Diagnostics (Basel). 2024. PMID: 38396398 Free PMC article. Review.
-
Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis Reveals Significant Ethnic Differences.ACS Chem Neurosci. 2020 Oct 7;11(19):2944-2961. doi: 10.1021/acschemneuro.0c00460. Epub 2020 Sep 17. ACS Chem Neurosci. 2020. PMID: 32870641 Free PMC article.
-
Prevalence, Patterns, Prognosis, and Psychosocial Impact of Olfactory and Gustative Dysfunctions Among Saudi COVID-19 Patients.Cureus. 2022 Nov 21;14(11):e31743. doi: 10.7759/cureus.31743. eCollection 2022 Nov. Cureus. 2022. PMID: 36569668 Free PMC article.
-
The Luminescence Hypothesis of Olfaction.Sensors (Basel). 2023 Jan 25;23(3):1333. doi: 10.3390/s23031333. Sensors (Basel). 2023. PMID: 36772376 Free PMC article.
-
Therapeutic Approaches to the Neurologic Manifestations of COVID-19.Neurotherapeutics. 2022 Sep;19(5):1435-1466. doi: 10.1007/s13311-022-01267-y. Epub 2022 Jul 21. Neurotherapeutics. 2022. PMID: 35861926 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

