DNA ligase I variants fail in the ligation of mutagenic repair intermediates with mismatches and oxidative DNA damage
- PMID: 32914844
- PMCID: PMC7846189
- DOI: 10.1093/mutage/geaa023
DNA ligase I variants fail in the ligation of mutagenic repair intermediates with mismatches and oxidative DNA damage
Abstract
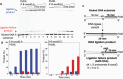
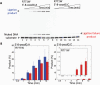
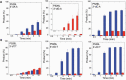
DNA ligase I (LIG1) joins DNA strand breaks during DNA replication and repair transactions and contributes to genome integrity. The mutations (P529L, E566K, R641L and R771W) in LIG1 gene are described in patients with LIG1-deficiency syndrome that exhibit immunodeficiency. LIG1 senses 3'-DNA ends with a mismatch or oxidative DNA base inserted by a repair DNA polymerase. However, the ligation efficiency of the LIG1 variants for DNA polymerase-promoted mutagenesis products with 3'-DNA mismatches or 8-oxo-2'-deoxyguanosine (8-oxodG) remains undefined. Here, we report that R641L and R771W fail in the ligation of nicked DNA with 3'-8-oxodG, leading to an accumulation of 5'-AMP-DNA intermediates in vitro. Moreover, we found that the presence of all possible 12 non-canonical base pairs variously impacts the ligation efficiency by P529L and R771W depending on the architecture at the DNA end, whereas E566K exhibits no activity against all substrates tested. Our results contribute to the understanding of the substrate specificity and mismatch discrimination of LIG1 for mutagenic repair intermediates and the effect of non-synonymous mutations on ligase fidelity.
© The Author(s) 2020. Published by Oxford University Press on behalf of the UK Environmental Mutagen Society.All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



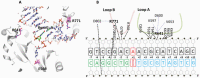





Similar articles
-
DNA ligase I fidelity mediates the mutagenic ligation of pol β oxidized and mismatch nucleotide insertion products in base excision repair.J Biol Chem. 2021 Jan-Jun;296:100427. doi: 10.1016/j.jbc.2021.100427. Epub 2021 Feb 16. J Biol Chem. 2021. PMID: 33600799 Free PMC article.
-
Mechanistic Basis for a Single Amino Acid Residue Mutation Causing Human DNA Ligase 1 Deficiency, A Rare Pediatric Disease.J Mol Biol. 2024 Nov 15;436(22):168813. doi: 10.1016/j.jmb.2024.168813. Epub 2024 Oct 5. J Mol Biol. 2024. PMID: 39374888
-
Rare variants of DNA ligase 1 show distinct mechanisms of deficiency.J Biol Chem. 2024 Dec;300(12):107957. doi: 10.1016/j.jbc.2024.107957. Epub 2024 Nov 5. J Biol Chem. 2024. PMID: 39510190 Free PMC article.
-
Alternative Okazaki Fragment Ligation Pathway by DNA Ligase III.Genes (Basel). 2015 Jun 23;6(2):385-98. doi: 10.3390/genes6020385. Genes (Basel). 2015. PMID: 26110316 Free PMC article. Review.
-
DNA Repair in mammalian cells: Mismatched repair: variations on a theme.Cell Mol Life Sci. 2009 Mar;66(6):1021-38. doi: 10.1007/s00018-009-8739-9. Cell Mol Life Sci. 2009. PMID: 19153655 Free PMC article. Review.
Cited by
-
DNA ligase I fidelity mediates the mutagenic ligation of pol β oxidized and mismatch nucleotide insertion products in base excision repair.J Biol Chem. 2021 Jan-Jun;296:100427. doi: 10.1016/j.jbc.2021.100427. Epub 2021 Feb 16. J Biol Chem. 2021. PMID: 33600799 Free PMC article.
-
Severe Combined Immunodeficiency from a Homozygous DNA Ligase 1 Mutant with Reduced Catalytic Activity but Increased Ligation Fidelity.J Clin Immunol. 2024 Jun 19;44(7):151. doi: 10.1007/s10875-024-01754-1. J Clin Immunol. 2024. PMID: 38896336 Free PMC article.
-
Case report: Severe combined immunodeficiency with ligase 1 deficiency and Omenn-like manifestation.Front Immunol. 2022 Oct 19;13:1033338. doi: 10.3389/fimmu.2022.1033338. eCollection 2022. Front Immunol. 2022. PMID: 36341401 Free PMC article.
-
Repair of Retrorsine-Induced DNA Damage in Rat Livers: Insights Gained from Transcriptomic and Proteomic Studies.Toxins (Basel). 2024 Dec 13;16(12):538. doi: 10.3390/toxins16120538. Toxins (Basel). 2024. PMID: 39728796 Free PMC article.
-
Targeting the Hippo Pathway in Breast Cancer: A Proteomic Analysis of Yes-Associated Protein Inhibition.Int J Mol Sci. 2025 Apr 22;26(9):3943. doi: 10.3390/ijms26093943. Int J Mol Sci. 2025. PMID: 40362184 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

