DNA ligase I variants fail in the ligation of mutagenic repair intermediates with mismatches and oxidative DNA damage
- PMID: 32914844
- PMCID: PMC7846189
- DOI: 10.1093/mutage/geaa023
DNA ligase I variants fail in the ligation of mutagenic repair intermediates with mismatches and oxidative DNA damage
Abstract
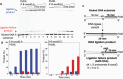
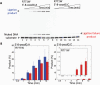
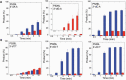
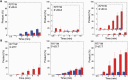
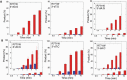
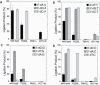
DNA ligase I (LIG1) joins DNA strand breaks during DNA replication and repair transactions and contributes to genome integrity. The mutations (P529L, E566K, R641L and R771W) in LIG1 gene are described in patients with LIG1-deficiency syndrome that exhibit immunodeficiency. LIG1 senses 3'-DNA ends with a mismatch or oxidative DNA base inserted by a repair DNA polymerase. However, the ligation efficiency of the LIG1 variants for DNA polymerase-promoted mutagenesis products with 3'-DNA mismatches or 8-oxo-2'-deoxyguanosine (8-oxodG) remains undefined. Here, we report that R641L and R771W fail in the ligation of nicked DNA with 3'-8-oxodG, leading to an accumulation of 5'-AMP-DNA intermediates in vitro. Moreover, we found that the presence of all possible 12 non-canonical base pairs variously impacts the ligation efficiency by P529L and R771W depending on the architecture at the DNA end, whereas E566K exhibits no activity against all substrates tested. Our results contribute to the understanding of the substrate specificity and mismatch discrimination of LIG1 for mutagenic repair intermediates and the effect of non-synonymous mutations on ligase fidelity.
© The Author(s) 2020. Published by Oxford University Press on behalf of the UK Environmental Mutagen Society.All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



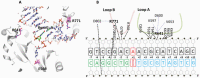





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

