Past, present, and future developments in enantioselective analysis using capillary electromigration techniques
- PMID: 32914880
- PMCID: PMC7821218
- DOI: 10.1002/elps.202000151
Past, present, and future developments in enantioselective analysis using capillary electromigration techniques
Abstract
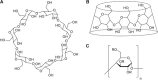
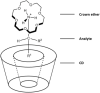
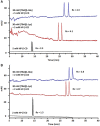
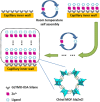
Enantioseparation of chiral products has become increasingly important in a large diversity of academic and industrial applications. The separation of chiral compounds is inherently challenging and thus requires a suitable analytical technique that can achieve high resolution and sensitivity. In this context, CE has shown remarkable results so far. Chiral CE offers an orthogonal enantioselectivity and is typically considered less costly than chromatographic techniques, since only minute amounts of chiral selectors are needed. Several CE approaches have been developed for chiral analysis, including chiral EKC and chiral CEC. Enantioseparations by EKC benefit from the wide variety of possible pseudostationary phases that can be employed. Chiral CEC, on the other hand, combines chromatographic separation principles with the bulk fluid movement of CE, benefitting from reduced band broadening as compared to pressure-driven systems. Although UV detection is conventionally used for these approaches, MS can also be considered. CE-MS represents a promising alternative due to the increased sensitivity and selectivity, enabling the chiral analysis of complex samples. The potential contamination of the MS ion source in EKC-MS can be overcome using partial-filling and counter-migration techniques. However, chiral analysis using monolithic and open-tubular CEC-MS awaits additional method validation and a dedicated commercial interface. Further efforts in chiral CE are expected toward the improvement of existing techniques, the development of novel pseudostationary phases, and establishing the use of chiral ionic liquids, molecular imprinted polymers, and metal-organic frameworks. These developments will certainly foster the adoption of CE(-MS) as a well-established technique in routine chiral analysis.
Keywords: CE-MS; Chiral CEC; Chiral analysis; Chiral electrokinetic chromatography; Enantioselective separation.
© 2020 The Authors. Electrophoresis published by Wiley-VCH GmbH.
Conflict of interest statement
Figures
Similar articles
-
[Advances in chiral separation and analysis by capillary electrophoresis-mass spectrometry].Se Pu. 2022 Jun;40(6):509-519. doi: 10.3724/SP.J.1123.2021.11006. Se Pu. 2022. PMID: 35616196 Free PMC article. Review. Chinese.
-
Capillary Electrophoresis Mass Spectrometry: Developments and Applications for Enantioselective Analysis from 2011-2020.Molecules. 2022 Jun 27;27(13):4126. doi: 10.3390/molecules27134126. Molecules. 2022. PMID: 35807372 Free PMC article. Review.
-
[Recent advance of novel chiral separation systems in capillary electrophoresis].Se Pu. 2020 Sep 8;38(9):1028-1037. doi: 10.3724/SP.J.1123.2020.02010. Se Pu. 2020. PMID: 34213269 Chinese.
-
Chiral Capillary Electrophoresis-Mass Spectrometry: Developments and Applications in the Period 2010-2015: A Review.J Chromatogr Sci. 2016 Nov;54(10):1771-1786. doi: 10.1093/chromsci/bmw100. Epub 2016 Jul 1. J Chromatogr Sci. 2016. PMID: 27371855 Review.
-
Chiral Capillary Electrophoresis-Mass Spectrometry.Methods Mol Biol. 2019;1985:391-405. doi: 10.1007/978-1-4939-9438-0_23. Methods Mol Biol. 2019. PMID: 31069748
Cited by
-
Comparison of the Performance of Different Bile Salts in Enantioselective Separation of Palonosetron Stereoisomers by Micellar Electrokinetic Chromatography.Molecules. 2022 Aug 16;27(16):5233. doi: 10.3390/molecules27165233. Molecules. 2022. PMID: 36014471 Free PMC article.
-
The Use of Dual Cyclodextrin Chiral Selector Systems in the Enantioseparation of Pharmaceuticals by Capillary Electrophoresis: An Overview.Molecules. 2021 Apr 14;26(8):2261. doi: 10.3390/molecules26082261. Molecules. 2021. PMID: 33919692 Free PMC article. Review.
-
Switching Separation Migration Order by Switching Electrokinetic Regime in Electrokinetic Microsystems.Biosensors (Basel). 2024 Feb 22;14(3):119. doi: 10.3390/bios14030119. Biosensors (Basel). 2024. PMID: 38534226 Free PMC article.
-
Chiral Capillary Electrokinetic Chromatography: Principle and Applications, Detection and Identification, Design of Experiment, and Exploration of Chiral Recognition Using Molecular Modeling.Molecules. 2021 May 11;26(10):2841. doi: 10.3390/molecules26102841. Molecules. 2021. PMID: 34064769 Free PMC article. Review.
-
Preparation of DNA nanoflower-modified capillary silica monoliths for chiral separation.Mikrochim Acta. 2024 Sep 9;191(10):584. doi: 10.1007/s00604-024-06663-z. Mikrochim Acta. 2024. PMID: 39245760
References
-
- Pasteur, L. , Comptes rendus Hebd. des séances l’ Académie des Sci. 1848, 26, 535–538.
-
- Schug, K. A. , Lindner, W. , J. Sep. Sci. 2005, 28, 1932–1955.
-
- Greenwood, D. R. , Comeskey, D. , Hunt, M. B. , Rasmussen, L. E. L. , Nature 2005, 438, 1097–1098. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

