Dinitrogen Fixation: Rationalizing Strategies Utilizing Molecular Complexes
- PMID: 32914919
- PMCID: PMC7986120
- DOI: 10.1002/chem.202003134
Dinitrogen Fixation: Rationalizing Strategies Utilizing Molecular Complexes
Abstract
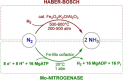
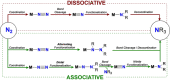
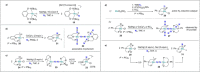
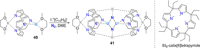
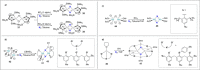
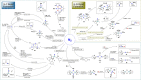
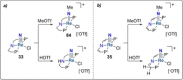
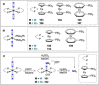
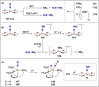
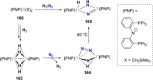
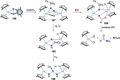
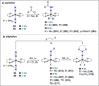
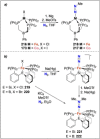
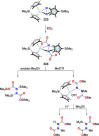
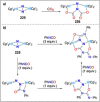
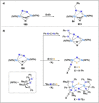
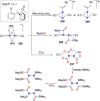
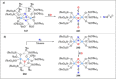
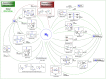
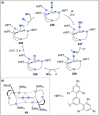
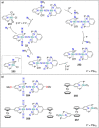
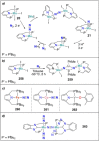
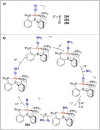
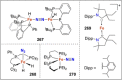
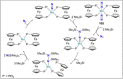
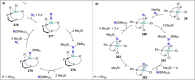
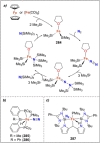
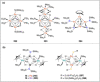
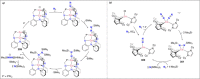
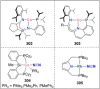
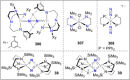
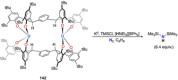
Dinitrogen (N2 ) is the most abundant gas in Earth's atmosphere, but its inertness hinders its use as a nitrogen source in the biosphere and in industry. Efficient catalysts are hence required to ov. ercome the high kinetic barriers associated to N2 transformation. In that respect, molecular complexes have demonstrated strong potential to mediate N2 functionalization reactions under mild conditions while providing a straightforward understanding of the reaction mechanisms. This Review emphasizes the strategies for N2 reduction and functionalization using molecular transition metal and actinide complexes according to their proposed reaction mechanisms, distinguishing complexes inducing cleavage of the N≡N bond before (dissociative mechanism) or concomitantly with functionalization (associative mechanism). We present here the main examples of stoichiometric and catalytic N2 functionalization reactions following these strategies.
Keywords: catalysis; coordination complexes; functionalization; mechanism; nitrogen.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
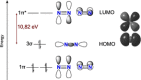


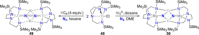
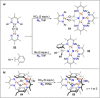
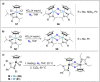
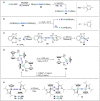
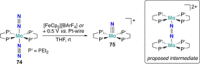






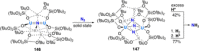
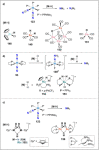
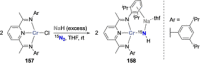
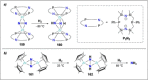





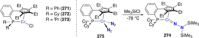

References
-
- Peoples M. B., Herridge D. F., Ladha J. K. in Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural Systems (Eds.: Ladha J. K., Peoples M. B.), Springer Netherlands, Dordrecht, 1995, pp. 3–28.
-
- F. Haber, The Synthesis of Ammonia from its Elements, Nobel Lecture, 1920.
-
- C. Bosch, The Development of the Chemical High Pressure Method During the Establishment of the New Ammonia Industry, Nobel Lecture, 1932.
-
- Smil V., Nature 1999, 400, 415.
-
- Smil V., Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production, Vol. 79, MIT Press, Cambridge, MA, 2001.
Publication types
LinkOut - more resources
Full Text Sources

