Cost-Effectiveness of an Antibacterial Envelope for Cardiac Implantable Electronic Device Infection Prevention in the US Healthcare System From the WRAP-IT Trial
- PMID: 32915063
- PMCID: PMC7566304
- DOI: 10.1161/CIRCEP.120.008503
Cost-Effectiveness of an Antibacterial Envelope for Cardiac Implantable Electronic Device Infection Prevention in the US Healthcare System From the WRAP-IT Trial
Abstract
Background: In the WRAP-IT trial (Worldwide Randomized Antibiotic Envelope Infection Prevention), adjunctive use of an absorbable antibacterial envelope resulted in a 40% reduction of major cardiac implantable electronic device infection without increased risk of complication in 6983 patients undergoing cardiac implantable electronic device revision, replacement, upgrade, or initial cardiac resynchronization therapy defibrillator implant. There is limited information on the cost-effectiveness of this strategy. As a prespecified objective, we evaluated antibacterial envelope cost-effectiveness compared with standard-of-care infection prevention strategies in the US healthcare system.
Methods: A decision tree model was used to compare costs and outcomes of antibacterial envelope (TYRX) use adjunctive to standard-of-care infection prevention versus standard-of-care alone over a lifelong time horizon. The analysis was performed from an integrated payer-provider network perspective. Infection rates, antibacterial envelope effectiveness, infection treatment costs and patterns, infection-related mortality, and utility estimates were obtained from the WRAP-IT trial. Life expectancy and long-term costs associated with device replacement, follow-up, and healthcare utilization were sourced from the literature. Costs and quality-adjusted life years were discounted at 3%. An upper willingness-to-pay threshold of $150 000 per quality-adjusted life year was used to determine cost-effectiveness, in alignment with the American College of Cardiology/American Heart Association practice guidelines and as supported by the World Health Organization and contemporary literature.
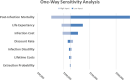
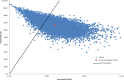
Results: The base case incremental cost-effectiveness ratio of the antibacterial envelope compared with standard-of-care was $112 603/quality-adjusted life year. The incremental cost-effectiveness ratio remained lower than the willingness-to-pay threshold in 74% of iterations in the probabilistic sensitivity analysis and was most sensitive to the following model inputs: infection-related mortality, life expectancy, and infection cost.
Conclusions: The absorbable antibacterial envelope was associated with a cost-effectiveness ratio below contemporary benchmarks in the WRAP-IT patient population, suggesting that the envelope provides value for the US healthcare system by reducing the incidence of cardiac implantable electronic device infection. Registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT02277990.
Keywords: defibrillators; incidence; infections; mortality; population.
Figures
References
-
- Sohail MR, Henrikson CA, Braid-Forbes MJ, Forbes KF, Lerner DJ. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med. 2011; 171:1821–1828. doi: 10.1001/archinternmed.2011.441 - PubMed
-
- Ahmed FZ, Fullwood C, Zaman M, Qamruddin A, Cunnington C, Mamas MA, Sandoe J, Motwani M, Zaidi A. Cardiac implantable electronic device (CIED) infections are expensive and associated with prolonged hospitalisation: UK retrospective observational study. PLoS One. 2019; 14:e0206611 doi: 10.1371/journal.pone.0206611 - PMC - PubMed
-
- Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B, Ayala-Paredes F, Tang AS, Sapp J, Sturmer M, et al. ; BRUISE CONTROL Investigators. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol. 2016; 67:1300–1308. doi: 10.1016/j.jacc.2016.01.009 - PubMed
-
- Wilkoff BL, Boriani G, Mittal S, Poole JE, Kennergren C, Corey GR, Love JC, Augostini R, Faerestrand S, Wiggins SS, et al. ; WRAP-IT Investigators. Impact of cardiac implantable electronic device infection: a clinical and economic analysis of the WRAP-IT trial. Circ Arrhythm Electrophysiol. 2020; 13:e008280 doi: 10.1161/CIRCEP.119.008280 - PMC - PubMed
-
- Tarakji KG, Mittal S, Kennergren C, Corey R, Poole J, Stromberg K, Lexcen DR, Wilkoff BL. Worldwide randomized antibiotic envelope infection prevention trial (WRAP-IT). Am Heart J. 2016; 180:12–21. doi: 10.1016/j.ahj.2016.06.010 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

