Increased number of pulmonary megakaryocytes in COVID-19 patients with diffuse alveolar damage: an autopsy study with clinical correlation and review of the literature
- PMID: 32915265
- PMCID: PMC7483503
- DOI: 10.1007/s00428-020-02926-1
Increased number of pulmonary megakaryocytes in COVID-19 patients with diffuse alveolar damage: an autopsy study with clinical correlation and review of the literature
Abstract
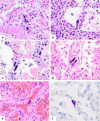
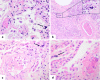
Pulmonary megakaryocytes participate in the pathogenesis of lung damage, particularly in acute lung injury. Although megakaryocytes are not mentioned as a characteristic histologic finding associated to pulmonary injury, a few studies reveal that their number is increased in diffuse alveolar damage (DAD). In this autopsy study, we have observed a relevant number of pulmonary megakaryocytes in COVID-19 patients dying with acute lung injury (7.61 ± 5.59 megakaryocytes per 25 high-power fields vs. 1.14 ± 0.86 for the control group, p < 0.05). We analyzed samples of 18 patients, most of whom died after prolonged disease and use of mechanical ventilation. Most patients showed advanced DAD and abnormal coagulation parameters with high levels of fibrinogen, D-dimers, and variable thrombocytopenia. For comparison, pulmonary samples from a group of 14 non-COVID-19 patients dying with DAD were reviewed. They showed similar pulmonary histopathologic findings and an increase in the number of megakaryocytes (4 ± 4.17 vs. 1.14 ± 0.86 for the control group, p < 0.05). Megakaryocyte count in the COVID-19 group was greater but did not reach statistical significance (7.61 ± 5.59 vs. 4 ± 4.17, p = 0.063). Regardless of the cause, pulmonary megakaryocytes are increased in patients with DAD. Their high number seen in COVID-19 patients suggests a relation with the thrombotic events so often seen these patients. Since the lung is considered an active site of megakaryopoiesis, a prothrombotic status leading to platelet activation, aggregation and consumption may trigger a compensatory pulmonary response.
Keywords: COVID-19; Diffuse alveolar damage; Megakaryocytes; Severe acute respiratory syndrome coronavirus 2; Thrombocytopenia; Thrombosis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Tracking the time course of pathological patterns of lung injury in severe COVID-19.Respir Res. 2021 Jan 29;22(1):32. doi: 10.1186/s12931-021-01628-9. Respir Res. 2021. PMID: 33514373 Free PMC article.
-
The Role of TLR-2 in Lethal COVID-19 Disease Involving Medullary and Resident Lung Megakaryocyte Up-Regulation in the Microthrombosis Mechanism.Cells. 2024 May 17;13(10):854. doi: 10.3390/cells13100854. Cells. 2024. PMID: 38786077 Free PMC article.
-
Thromboembolic involvement and its possible pathogenesis in COVID-19 mortality: lesson from post-mortem reports.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1670-1679. doi: 10.26355/eurrev_202102_24878. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629337 Review.
-
A proof of evidence supporting abnormal immunothrombosis in severe COVID-19: naked megakaryocyte nuclei increase in the bone marrow and lungs of critically ill patients.Platelets. 2020 Nov 16;31(8):1085-1089. doi: 10.1080/09537104.2020.1810224. Epub 2020 Aug 28. Platelets. 2020. PMID: 32857624
-
The pulmonary pathology of COVID-19.Virchows Arch. 2021 Jan;478(1):137-150. doi: 10.1007/s00428-021-03053-1. Epub 2021 Feb 19. Virchows Arch. 2021. PMID: 33604758 Free PMC article. Review.
Cited by
-
Cardio-Pulmonary Histopathology with Clinical Correlations of Deceased Patients with COVID-19: A Case Series in Tehran, Iran.Arch Iran Med. 2023 May 1;26(5):252-260. doi: 10.34172/aim.2023.39. Arch Iran Med. 2023. PMID: 38301088 Free PMC article.
-
Immune and Inflammatory Properties of Megakaryocytes.Cells. 2025 Jul 10;14(14):1053. doi: 10.3390/cells14141053. Cells. 2025. PMID: 40710306 Free PMC article. Review.
-
The Role of Von Willebrand Factor in the Pathogenesis of Pulmonary Vascular Thrombosis in COVID-19.Viruses. 2022 Jan 21;14(2):211. doi: 10.3390/v14020211. Viruses. 2022. PMID: 35215805 Free PMC article.
-
Meta-Analysis and Systematic Review of Coagulation Disbalances in COVID-19: 41 Studies and 17,601 Patients.Front Cardiovasc Med. 2022 Mar 11;9:794092. doi: 10.3389/fcvm.2022.794092. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35360017 Free PMC article.
-
Postmortem findings in COVID-19 fatalities: A systematic review of current evidence.Leg Med (Tokyo). 2022 Feb;54:102001. doi: 10.1016/j.legalmed.2021.102001. Epub 2021 Dec 7. Leg Med (Tokyo). 2022. PMID: 34952452 Free PMC article.
References
-
- Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegué E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. - DOI - PMC - PubMed
-
- Cheung O-Y, Graziano P, Smith ML (2018) Acute lung injury. In: Leslie KO, Wick MR (eds) Practical pulmonary pathology: a diagnostic approach, 3rd edn. Elsevier, Philadelphia, pp 125–146
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

