A nomogram based on pretreatment clinical parameters for the prediction of inadequate biochemical response in primary biliary cholangitis
- PMID: 32915500
- PMCID: PMC7676192
- DOI: 10.1002/jcla.23501
A nomogram based on pretreatment clinical parameters for the prediction of inadequate biochemical response in primary biliary cholangitis
Abstract
Background: Ursodeoxycholic acid (UDCA) has been widely recommended as the first-line drug for primary biliary cholangitis (PBC) in the current guidelines. However, its therapeutic effects are poor in nearly one-third of patients. The early identification and intervention of these patients is crucial for delaying disease progression. Therefore, we explored risk factors for inadequate biochemical response and constructed a nomogram to predict the potential risk.
Methods: We enrolled 356 patients and randomly divided them into training (70%) and validation groups (30%). We defined inadequate biochemical response as the study endpoint. Logistic analysis was used to identify the independent predictors of poor biochemical response. Based on these factors, a predictive nomogram was finally constructed. Then, discrimination and calibration were evaluated by internal validation. Additionally, the association between the model predictions and prognosis was further analyzed.
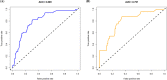
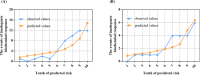
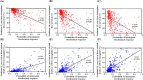
Results: Female sex, and albumin and bilirubin concentrations were identified as risk factors, and a nomogram was built based on these factors. The areas under the ROC curves of the training and validation groups were 0.809 and 0.791, respectively. Moreover, calibration curves showed that predictions of the nomogram had good concordance with the actual outcomes. The correlation analysis demonstrated that PBC patients with a high probability of a suboptimal biochemical response were more likely to have adverse outcomes.
Conclusion: We constructed a nomogram, which can accurately predict the risk of inadequate biochemical response to UDCA, facilitating the early screening of high-risk patients with PBC who should be prioritized for additional therapy.
Keywords: autoimmune liver disease; biochemical response; nomogram; primary biliary cholangitis; ursodeoxycholic acid.
© 2020 The Authors. Journal of Clinical Laboratory Analysis Published by Wiley Periodicals, Inc.
Figures

References
-
- Gulamhusein AF, Hirschfield GM. Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat Rev Gastroenterol Hepatol. 2020;17(2):93‐110. - PubMed
-
- Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015;386(10003):1565‐1575. - PubMed
-
- Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon R‐E, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128(2):297‐303. - PubMed
-
- Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long‐term prognosis in primary biliary cirrhosis. Hepatology. 2008;48(3):871‐877. - PubMed
MeSH terms
Substances
Grants and funding
- 81770569/National Natural Science Foundation of China
- 81870421/National Natural Science Foundation of China
- 2018BSHYDZZ74/Postdoctoral Science Foundation of Shaanxi Province
- 2018M643871/China Postdoctoral Science Foundation
- 81820108005/International Cooperation and Exchange of the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources

