Mechanisms Targeting the Unfolded Protein Response in Asthma
- PMID: 32915643
- PMCID: PMC12042654
- DOI: 10.1165/rcmb.2019-0235TR
Mechanisms Targeting the Unfolded Protein Response in Asthma
Abstract
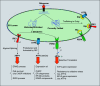
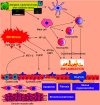
Lung cells are constantly exposed to various internal and external stressors that disrupt protein homeostasis. To cope with these stimuli, cells evoke a highly conserved adaptive mechanism called the unfolded protein response (UPR). UPR stressors can impose greater protein secretory demands on the endoplasmic reticulum (ER), resulting in the development, differentiation, and survival of these cell types to meet these increasing functional needs. Dysregulation of the UPR leads to the development of the disease. The UPR and ER stress are involved in several human conditions, such as chronic inflammation, neurodegeneration, metabolic syndrome, and cancer. Furthermore, potent and specific compounds that target the UPR pathway are under development as future therapies. The focus of this review is to thoroughly describe the effects of both internal and external stressors on the ER in asthma. Furthermore, we discuss how the UPR signaling pathway is activated in the lungs to overcome cellular damage. We also present an overview of the pathogenic mechanisms, with a brief focus on potential strategies for pharmacological interventions.
Keywords: asthma; endoplasmic reticulum; endoplasmic reticulum stress; unfolded protein response.
Figures
References
-
- Duvigneau JC, Luís A, Gorman AM, Samali A, Kaltenecker D, Moriggl R, et al. Crosstalk between inflammatory mediators and endoplasmic reticulum stress in liver diseases. Cytokine . 2019;124:154577. - PubMed
-
- Ozgur R, Uzilday B, Iwata Y, Koizumi N, Turkan I. Interplay between the unfolded protein response and reactive oxygen species: a dynamic duo. J Exp Bot . 2018;69:3333–3345. - PubMed
-
- Manalo RVM, Medina PMB. The endoplasmic reticulum stress response in disease pathogenesis and pathophysiology. Egypt J Med Hum Genet . 2018;19:59–68.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

