Activity of transgene-produced B-domain-deleted factor VIII in human plasma following AAV5 gene therapy
- PMID: 32915950
- PMCID: PMC7714098
- DOI: 10.1182/blood.2020005683
Activity of transgene-produced B-domain-deleted factor VIII in human plasma following AAV5 gene therapy
Abstract
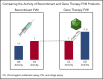
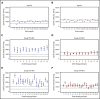
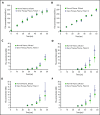
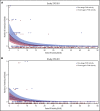
Adeno-associated virus (AAV)-based gene therapies can restore endogenous factor VIII (FVIII) expression in hemophilia A (HA). AAV vectors typically use a B-domain-deleted FVIII transgene, such as human FVIII-SQ in valoctocogene roxaparvovec (AAV5-FVIII-SQ). Surprisingly, the activity of transgene-produced FVIII-SQ was between 1.3 and 2.0 times higher in one-stage clot (OS) assays than in chromogenic-substrate (CS) assays, whereas recombinant FVIII-SQ products had lower OS than CS activity. Transgene-produced and recombinant FVIII-SQ showed comparable specific activity (international units per milligram) in the CS assay, demonstrating that the diverging activities arise in the OS assay. Higher OS activity for transgene-produced FVIII-SQ was observed across various assay kits and clinical laboratories, suggesting that intrinsic molecular features are potential root causes. Further experiments in 2 participants showed that transgene-produced FVIII-SQ accelerated early factor Xa and thrombin formation, which may explain the higher OS activity based on a kinetic bias between OS and CS assay readout times. Despite the faster onset of coagulation, global thrombin levels were unaffected. A correlation with joint bleeds suggested that both OS and CS assay remained clinically meaningful to distinguish hemophilic from nonhemophilic FVIII activity levels. During clinical development, the CS activity was chosen as a surrogate end point to conservatively assess hemostatic efficacy and enable comparison with recombinant FVIII-SQ products. Relevant trials are registered on clinicaltrials.gov as #NCT02576795 and #NCT03370913 and, respectively, on EudraCT (European Union Drug Regulating Authorities Clinical Trials Database; https://eudract.ema.europa.eu) as #2014-003880-38 and #2017-003215-19.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: S. Rosen is chairman of Rossix AB. S.T. and M.R. are employees and hold stock in Laboratory Corporation of America Holdings. S. Rosen and G.F.P. are consultants for BioMarin Pharmaceutical Inc. M.H., J.S., D.M., T.C., B.K., S.J.Z., and C.V. are employees and own stock in BioMarin Pharmaceutical Inc. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Gene-produced FVIII: measure for measure.Blood. 2020 Nov 26;136(22):2483-2484. doi: 10.1182/blood.2020008974. Blood. 2020. PMID: 33242331 No abstract available.
References
-
- Rangarajan S, Walsh L, Lester W, et al. . AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N Engl J Med. 2017;377(26):2519-2530. - PubMed
-
- Mikaelsson M, Oswaldsson U, Sandberg H. Influence of phospholipids on the assessment of factor VIII activity. Haemophilia. 1998;4(4):646-650. - PubMed
-
- Barrowcliffe TW, Raut S, Hubbard AR. Discrepancies in potency assessment of recombinant FVIII concentrates. Haemophilia. 1998;4(4):634-640. - PubMed
-
- Sandberg H, Kannicht C, Stenlund P, et al. . Functional characteristics of the novel, human-derived recombinant FVIII protein product, human-cl rhFVIII. Thromb Res. 2012;130(5):808-817. - PubMed
-
- Hubbard AR, Weller LJ, Bevan SA. A survey of one-stage and chromogenic potencies in therapeutic factor VIII concentrates. Br J Haematol. 2002;117(1):247-248. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

