Ibrutinib added to 10-day decitabine for older patients with AML and higher risk MDS
- PMID: 32915972
- PMCID: PMC7509861
- DOI: 10.1182/bloodadvances.2020002846
Ibrutinib added to 10-day decitabine for older patients with AML and higher risk MDS
Abstract
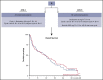
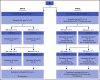
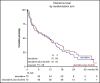
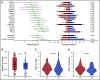
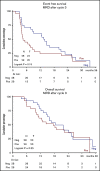
The treatment of older, unfit patients with acute myeloid leukemia (AML) is challenging. Based on preclinical data of Bruton tyrosine kinase expression/phosphorylation and ibrutinib cytotoxicity in AML blasts, we conducted a randomized phase 2 multicenter study to assess the tolerability and efficacy of the addition of ibrutinib to 10-day decitabine in unfit (ie, Hematopoietic Cell Transplantation Comorbidity Index ≥3) AML patients and higher risk myelodysplasia patients (HOVON135/SAKK30/15 trial). In total, 144 eligible patients were randomly (1:1) assigned to either 10-day decitabine combined with ibrutinib (560 mg; sequentially given, starting the day after the last dose of decitabine) (n = 72) or to 10-day decitabine (n = 72). The addition of ibrutinib was well tolerated, and the number of adverse events was comparable for both arms. In the decitabine plus ibrutinib arm, 41% reached complete remission/complete remission with incomplete hematologic recovery (CR/CRi), the median overall survival (OS) was 11 months, and 2-year OS was 27%; these findings compared with 50% CR/CRi, median OS of 11.5 months, and 2-year OS of 21% for the decitabine group (not significant). Extensive molecular profiling at diagnosis revealed that patients with STAG2, IDH2, and ASXL1 mutations had significantly lower CR/CRi rates, whereas patients with mutations in TP53 had significantly higher CR/CRi rates. Furthermore, multicolor flow cytometry revealed that after 3 cycles of treatment, 28 (49%) of 57 patients with available bone marrow samples had no measurable residual disease. In this limited number of cases, measurable residual disease revealed no apparent impact on event-free survival and OS. In conclusion, the addition of ibrutinib does not improve the therapeutic efficacy of decitabine. This trial was registered at the Netherlands Trial Register (NL5751 [NTR6017]) and has EudraCT number 2015-002855-85.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: G.H. has been been involved in advisory boards for Janssen Pharmaceutics. The remaining authors declare no competing financial interests.
Figures

References
-
- Juliusson G, Antunovic P, Derolf A, et al. . Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187. - PubMed
-
- Ossenkoppele G, Löwenberg B. How I treat the older patient with acute myeloid leukemia. Blood. 2015;125(5):767-774. - PubMed
-
- Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. . Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562-569. - PubMed
-
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. . Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

