The Structures of Gd(III) Chelates Conjugated at the Periphery of 3-(1'-Hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH) Have a Significant Impact on the Imaging and Therapy of Cancer
- PMID: 32916033
- PMCID: PMC7722155
- DOI: 10.1002/cmdc.202000449
The Structures of Gd(III) Chelates Conjugated at the Periphery of 3-(1'-Hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH) Have a Significant Impact on the Imaging and Therapy of Cancer
Abstract
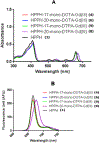
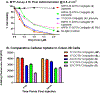
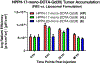
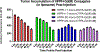
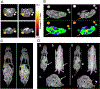
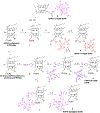
3-(1'-Hexyloxyethyl)-3-devinyl-pyropheophorbide-a (HPPH or Photochlor), a tumor-avid chlorophyll-a derivative currently undergoing human clinical trials, was conjugated at various peripheral positions (position-17 or 20) of HPPH with either Gd(III)-aminobenzyl-DTPA (Gd(III) DTPA) or Gd(III)-aminoethylamido-DOTA (Gd(III) DOTA). The corresponding conjugates were evaluated for in vitro PDT efficacy, T1 , T2 relaxivities, in vivo fluorescence, and MR imaging under similar treatment parameters. Among these analogs, the water-soluble Gd(III)-aminoethylamido-DOTA linked at position-17 of HPPH, i. e., HPPH-17-Gd(III) DOTA, demonstrated strong potential for tumor imaging by both MR and fluorescence, while maintaining the PDT efficacy in BALB/c mice bearing Colon-26 tumors (7/10 mice were tumor free on day 60). In contrast to Gd(III) DTPA (Magnevist) and Gd(III) DOTA (Dotarem), the HPPH-Gd(III) DOTA retains in the tumor for a long period of time (24 to 48 h) and provides an option of fluorescence-guided cancer therapy. Thus, a single agent can be used for cancer-imaging and therapy. However, further detailed pharmacokinetic, pharmacodynamic, and toxicological studies of the conjugate are required before initiating Phase I human clinical trials.
© 2020 Wiley-VCH GmbH.
Figures
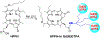

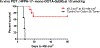
Similar articles
-
Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications.Int J Mol Sci. 2023 Dec 12;24(24):17404. doi: 10.3390/ijms242417404. Int J Mol Sci. 2023. PMID: 38139233 Free PMC article. Review.
-
Tumor-Avid 3-(1'-Hexyloxy)ethyl-3-devinylpyrpyropheophorbide-a (HPPH)-3Gd(III)tetraxetan (DOTA) Conjugate Defines Primary Tumors and Metastases.J Med Chem. 2022 Jul 14;65(13):9267-9280. doi: 10.1021/acs.jmedchem.2c00547. Epub 2022 Jun 28. J Med Chem. 2022. PMID: 35763292
-
Chlorophyll-a analogues conjugated with aminobenzyl-DTPA as potential bifunctional agents for magnetic resonance imaging and photodynamic therapy.Bioconjug Chem. 2005 Jan-Feb;16(1):32-42. doi: 10.1021/bc049807x. Bioconjug Chem. 2005. PMID: 15656573
-
Synthesis of Tumor-avid Photosensitizer-Gd(III)DTPA conjugates: impact of the number of gadolinium units in T1/T2 relaxivity, intracellular localization, and photosensitizing efficacy.Bioconjug Chem. 2010 May 19;21(5):816-27. doi: 10.1021/bc9005305. Bioconjug Chem. 2010. PMID: 20387863 Free PMC article.
-
Mesoscopic fluorescence tomography of a photosensitizer (HPPH) 3D biodistribution in skin cancer.Acad Radiol. 2014 Feb;21(2):271-80. doi: 10.1016/j.acra.2013.11.009. Acad Radiol. 2014. PMID: 24439340 Review.
Cited by
-
Theranostics with photodynamic therapy for personalized medicine: to see and to treat.Theranostics. 2023 Oct 9;13(15):5501-5544. doi: 10.7150/thno.87363. eCollection 2023. Theranostics. 2023. PMID: 37908729 Free PMC article. Review.
-
Photodynamic Therapy in Combination with Doxorubicin Is Superior to Monotherapy for the Treatment of Lung Cancer.Biomedicines. 2022 Apr 6;10(4):857. doi: 10.3390/biomedicines10040857. Biomedicines. 2022. PMID: 35453607 Free PMC article.
-
Liposomal Fe(III) Macrocyclic Complexes with Hydroxypropyl Pendants as MRI Probes.ACS Appl Bio Mater. 2021 Nov 15;4(11):7951-7960. doi: 10.1021/acsabm.1c00879. Epub 2021 Oct 15. ACS Appl Bio Mater. 2021. PMID: 35006776 Free PMC article.
-
Lanthanide porphyrinoids as molecular theranostics.Chem Soc Rev. 2022 Jul 18;51(14):6177-6209. doi: 10.1039/d2cs00275b. Chem Soc Rev. 2022. PMID: 35792133 Free PMC article. Review.
-
Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications.Int J Mol Sci. 2023 Dec 12;24(24):17404. doi: 10.3390/ijms242417404. Int J Mol Sci. 2023. PMID: 38139233 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

