Mosaicism in Human Health and Disease
- PMID: 32916079
- PMCID: PMC8483770
- DOI: 10.1146/annurev-genet-041720-093403
Mosaicism in Human Health and Disease
Abstract
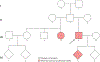
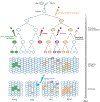
Mosaicism refers to the occurrence of two or more genomes in an individual derived from a single zygote. Germline mosaicism is a mutation that is limited to the gonads and can be transmitted to offspring. Somatic mosaicism is a postzygotic mutation that occurs in the soma, and it may occur at any developmental stage or in adult tissues. Mosaic variation may be classified in six ways: (a) germline or somatic origin, (b) class of DNA mutation (ranging in scale from single base pairs to multiple chromosomes), (c) developmental context, (d) body location(s), (e) functional consequence (including deleterious, neutral, or advantageous), and (f) additional sources of mosaicism, including mitochondrial heteroplasmy, exogenous DNA sources such as vectors, and epigenetic changes such as imprinting and X-chromosome inactivation. Technological advances, including single-cell and other next-generation sequencing, have facilitated improved sensitivity and specificity to detect mosaicism in a variety of biological contexts.
Keywords: germline mutation; mosaic aneuploidy; mosaicism; somatic mutation.
Figures


References
-
- Antonarakis SE. 2017. Down syndrome and the complexity of genome dosage imbalance. Nat. Rev. Genet 18:147–63 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

