Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals
- PMID: 32916100
- PMCID: PMC7855478
- DOI: 10.1016/S1473-3099(20)30477-1
Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals
Abstract
Background: The prevalence and effects of inappropriate empirical antibiotic therapy for bloodstream infections are unclear. We aimed to establish the population-level burden, predictors, and mortality risk of in-vitro susceptibility-discordant empirical antibiotic therapy among patients with bloodstream infections.
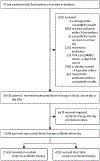
Methods: Our retrospective cohort analysis of electronic health record data from 131 hospitals in the USA included patients with suspected-and subsequently confirmed-bloodstream infections who were treated empirically with systemic antibiotics between Jan 1, 2005, and Dec 31, 2014. We included all patients with monomicrobial bacteraemia caused by common bloodstream pathogens who received at least one systemic antibiotic either on the day blood cultures were drawn or the day after, and for whom susceptibility data were available. We calculated the prevalence of discordant empirical antibiotic therapy-which was defined as receiving antibiotics on the day blood culture samples were drawn to which the cultured isolate was not susceptible in vitro-overall and by hospital type by using regression tree analysis. We used generalised estimating equations to identify predictors of receiving discordant empirical antibiotic therapy, and used logistic regression to calculate adjusted odds ratios for the relationship between in-hospital mortality and discordant empirical antibiotic therapy.
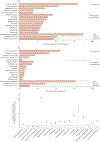
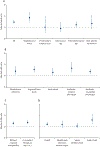
Findings: 21 608 patients with bloodstream infections received empirical antibiotic therapy on the day of first blood culture collection. Of these patients, 4165 (19%) received discordant empirical antibiotic therapy. Discordant empirical antibiotic therapy was independently associated with increased risk of mortality (adjusted odds ratio 1·46 [95% CI, 1·28-1·66]; p<0·0001), a relationship that was unaffected by the presence or absence of resistance or sepsis or septic shock. Infection with antibiotic-resistant species strongly predicted receiving discordant empirical therapy (adjusted odds ratio 9·09 [95% CI 7·68-10·76]; p<0·0001). Most incidences of discordant empirical antibiotic therapy and associated deaths occurred among patients with bloodstream infections caused by Staphylococcus aureus or Enterobacterales.
Interpretation: Approximately one in five patients with bloodstream infections in US hospitals received discordant empirical antibiotic therapy, receipt of which was closely associated with infection with antibiotic-resistant pathogens. Receiving discordant empirical antibiotic therapy was associated with increased odds of mortality overall, even in patients without sepsis. Early identification of bloodstream pathogens and resistance will probably improve population-level outcomes.
Funding: US National Institutes of Health, US Centers for Disease Control and Prevention, and US Agency for Healthcare Research and Quality.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Electronic health record data for antimicrobial prescribing.Lancet Infect Dis. 2021 Feb;21(2):155-157. doi: 10.1016/S1473-3099(20)30453-9. Epub 2020 Sep 8. Lancet Infect Dis. 2021. PMID: 32916099 No abstract available.
References
-
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017; 45(3): 486–552. - PubMed
-
- Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. JAntimicrob Chemother 2014; 69(4): 881–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

