Warmth Prevents Bone Loss Through the Gut Microbiota
- PMID: 32916104
- PMCID: PMC7116155
- DOI: 10.1016/j.cmet.2020.08.012
Warmth Prevents Bone Loss Through the Gut Microbiota
Abstract
Osteoporosis is the most prevalent metabolic bone disease, characterized by low bone mass and microarchitectural deterioration. Here, we show that warmth exposure (34°C) protects against ovariectomy-induced bone loss by increasing trabecular bone volume, connectivity density, and thickness, leading to improved biomechanical bone strength in adult female, as well as in young male mice. Transplantation of the warm-adapted microbiota phenocopies the warmth-induced bone effects. Both warmth and warm microbiota transplantation revert the ovariectomy-induced transcriptomics changes of the tibia and increase periosteal bone formation. Combinatorial metagenomics/metabolomics analysis shows that warmth enhances bacterial polyamine biosynthesis, resulting in higher total polyamine levels in vivo. Spermine and spermidine supplementation increases bone strength, while inhibiting polyamine biosynthesis in vivo limits the beneficial warmth effects on the bone. Our data suggest warmth exposure as a potential treatment option for osteoporosis while providing a mechanistic framework for its benefits in bone disease.
Keywords: bone; metabolomics; metadata; metagenomics; microbiota; osteoporosis; ovariectomy; polyamines; post-menopause; warm.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests M.T. and C.C. disclose that they are inventors of a submitted patent application for treatment of bone diseases. All other authors declare no competing interests.
Figures
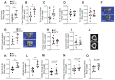


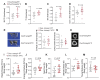
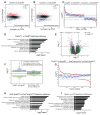


Comment in
-
Warmth prevents bone loss.Nat Rev Endocrinol. 2020 Dec;16(12):679. doi: 10.1038/s41574-020-00424-7. Nat Rev Endocrinol. 2020. PMID: 32963341 No abstract available.
-
Getting Warmer: Following One's Gut to Build Bone.Cell Metab. 2020 Oct 6;32(4):504-506. doi: 10.1016/j.cmet.2020.09.010. Cell Metab. 2020. PMID: 33027670 Free PMC article.
References
-
- Allen J. The influence of Physical conditions in the genesis of species. Radical Review. 1877;1:108–140.
-
- Ashoub MA. Effect of two extreme temperatures on growth and tail-length of mice. Nature. 1958;181:284. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

