FGFR3 in Periosteal Cells Drives Cartilage-to-Bone Transformation in Bone Repair
- PMID: 32916123
- PMCID: PMC7561512
- DOI: 10.1016/j.stemcr.2020.08.005
FGFR3 in Periosteal Cells Drives Cartilage-to-Bone Transformation in Bone Repair
Abstract
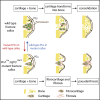
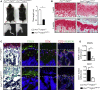
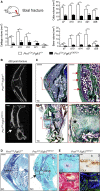
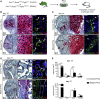
Most organs and tissues in the body, including bone, can repair after an injury due to the activation of endogenous adult stem/progenitor cells to replace the damaged tissue. Inherent dysfunctions of the endogenous stem/progenitor cells in skeletal repair disorders are still poorly understood. Here, we report that Fgfr3Y637C/+ over-activating mutation in Prx1-derived skeletal stem/progenitor cells leads to failure of fracture consolidation. We show that periosteal cells (PCs) carrying the Fgfr3Y637C/+ mutation can engage in osteogenic and chondrogenic lineages, but following transplantation do not undergo terminal chondrocyte hypertrophy and transformation into bone causing pseudarthrosis. Instead, Prx1Cre;Fgfr3Y637C/+ PCs give rise to fibrocartilage and fibrosis. Conversely, wild-type PCs transplanted at the fracture site of Prx1Cre;Fgfr3Y637C/+ mice allow hypertrophic cartilage transition to bone and permit fracture consolidation. The results thus highlight cartilage-to-bone transformation as a necessary step for bone repair and FGFR3 signaling within PCs as a key regulator of this transformation.
Keywords: FGFR3; bone repair; endochondral ossification; periosteum; pseudarthrosis; skeletal stem/progenitor cell.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Abou-Khalil R., Yang F., Lieu S., Julien A., Perry J., Pereira C., Relaix F., Miclau T., Marcucio R., Colnot C. Role of muscle stem cells during skeletal regeneration. Stem Cells. 2015;33:1501–1511. - PubMed
-
- Bonaventure J., Rousseau F., Legeai-Mallet L., le Merrer M., Munnich A., Maroteaux P. Common mutations in the fibroblast growth factor receptor 3 (FGFR 3) gene account for achondroplasia, hypochondroplasia, and thanatophoric dwarfism. Am. J. Med. Genet. 1996;63:148–154. - PubMed
-
- Chen H., Sun X., Yin L., Chen S., Zhu Y., Huang J., Jiang W., Chen B., Zhang R., Chen L. PTH 1-34 ameliorates the osteopenia and delayed healing of stabilized tibia fracture in mice with achondroplasia resulting from gain-of-function mutation of FGFR3. Int. J. Biol. Sci. 2017;13:1254–1265. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

