Biological behaviors of mutant proinsulin contribute to the phenotypic spectrum of diabetes associated with insulin gene mutations
- PMID: 32916194
- PMCID: PMC7734662
- DOI: 10.1016/j.mce.2020.111025
Biological behaviors of mutant proinsulin contribute to the phenotypic spectrum of diabetes associated with insulin gene mutations
Abstract
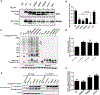
Insulin gene mutation is the second most common cause of neonatal diabetes (NDM). It is also one of the genes involved in maturity-onset diabetes of the young (MODY). We aim to investigate molecular behaviors of different INS gene variants that may correlate with the clinical spectrum of diabetes phenotypes. In this study, we concentrated on two previously uncharacterized MODY-causing mutants, proinsulin-p.Gly44Arg [G(B20)R] and p.Pro52Leu [P(B28)L] (a novel mutant identified in one French family), and an NDM causing proinsulin-p.(Cys96Tyr) [C(A7)Y]. We find that these proinsulin mutants exhibit impaired oxidative folding in the endoplasmic reticulum (ER) with blocked ER export, ER stress, and apoptosis. Importantly, the proinsulin mutants formed abnormal intermolecular disulfide bonds that not only involved the mutant proinsulin, but also the co-expressed WT-proinsulin, forming misfolded disulfide-linked proinsulin complexes. This impaired the intracellular trafficking of WT-proinsulin and limited the production of bioactive mature insulin. Notably, although all three mutants presented with similar defects in folding, trafficking, and dominant negative behavior, the degrees of these defects appeared to be different. Specifically, compared to MODY mutants G(B20)R and P(B28)L that partially affected folding and trafficking of co-expressed WT-proinsulin, the NDM mutant C(A7)Y resulted in an almost complete blockade of the ER export of WT-proinsulin, decreasing insulin production, inducing more severe ER stress and apoptosis. We thus demonstrate that differences in cell biological behaviors among different proinsulin mutants correlate with the spectrum of diabetes phenotypes caused by the different INS gene mutations.
Keywords: Dominant negative effect; ER stress; Insulin gene mutations; Maturity onset diabetes of the young; Neonatal diabetes mellitus; Proinsulin misfolding.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest
None.
Figures


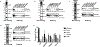

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

