Self-Collected versus Healthcare Worker-Collected Swabs in the Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2
- PMID: 32916801
- PMCID: PMC7554687
- DOI: 10.3390/diagnostics10090678
Self-Collected versus Healthcare Worker-Collected Swabs in the Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2
Abstract
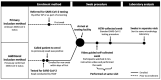
The aim of this study was to compare the sensitivity of self-collected versus healthcare worker (HCW)-collected swabs for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) testing. Symptomatic individuals referred for SARS-CoV-2 testing were invited to provide mobile-phone video-instructed self-collected oropharyngeal and nasal samples followed by a HCW-collected oropharyngeal sample. All samples were sent for analysis to the same microbiology laboratory, and the number of SARS-CoV-2-positive participants in the two tests was compared. A total of 109 participants were included, and 19 participants had SARS-CoV-2-positive results. The diagnostic sensitivity of the self-collected and HCW-collected swabs was 84.2% and 89.5%, respectively, with an acceptable agreement, Cohens kappa 0.82, p < 0.001. Further, results from a questionnaire answered by the participants found that loss of smell as a self-reported symptom was a strong predictor for a SARS-CoV-2-positive test. In conclusion, we found that self-collected oropharyngeal and nasal swabs for SARS-CoV-2 testing can be reliable compared to HCW-collected oropharyngeal samples.
Keywords: COVID-19; COVID-19 diagnostic testing; severe acute respiratory syndrome coronavirus 2.
Conflict of interest statement
Swabs from Zymo Collection Swab, Zymo Research, Irvine, CA, USA were given free of charge by Nordic Biosite, 1301 Copenhagen, Denmark. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
A prospective diagnostic evaluation of accuracy of self-taken and healthcare worker-taken swabs for rapid COVID-19 testing.PLoS One. 2022 Jun 30;17(6):e0270715. doi: 10.1371/journal.pone.0270715. eCollection 2022. PLoS One. 2022. PMID: 35771760 Free PMC article.
-
Performance Analysis of Self-Collected Nasal and Oral Swabs for Detection of SARS-CoV-2.Diagnostics (Basel). 2022 Sep 21;12(10):2279. doi: 10.3390/diagnostics12102279. Diagnostics (Basel). 2022. PMID: 36291968 Free PMC article.
-
The accuracy of healthcare worker versus self collected (2-in-1) Oropharyngeal and Bilateral Mid-Turbinate (OPMT) swabs and saliva samples for SARS-CoV-2.PLoS One. 2020 Dec 16;15(12):e0244417. doi: 10.1371/journal.pone.0244417. eCollection 2020. PLoS One. 2020. PMID: 33326503 Free PMC article.
-
Accuracy of a Novel SARS-CoV-2 Antigen-Detecting Rapid Diagnostic Test from Standardized Self-Collected Anterior Nasal Swabs.J Clin Med. 2021 May 13;10(10):2099. doi: 10.3390/jcm10102099. J Clin Med. 2021. PMID: 34068236 Free PMC article.
-
Performance of at-home self-collected saliva and nasal-oropharyngeal swabs in the surveillance of COVID-19.J Oral Microbiol. 2020 Dec 9;13(1):1858002. doi: 10.1080/20002297.2020.1858002. J Oral Microbiol. 2020. PMID: 33391631 Free PMC article.
Cited by
-
COVID-19 Rapid Antigen Tests With Self-Collected vs Health Care Worker-Collected Nasal and Throat Swab Specimens: A Randomized Clinical Trial.JAMA Netw Open. 2023 Dec 1;6(12):e2344295. doi: 10.1001/jamanetworkopen.2023.44295. JAMA Netw Open. 2023. PMID: 38055280 Free PMC article. Clinical Trial.
-
Different Respiratory Samples for COVID-19 Detection by Standard and Direct Quantitative RT-PCR: A Literature Review.Iran J Pharm Res. 2021 Summer;20(3):285-299. doi: 10.22037/ijpr.2021.115458.15383. Iran J Pharm Res. 2021. PMID: 34903989 Free PMC article. Review.
-
Danish citizens' preferences for at-home oropharyngeal/nasal SARS-CoV-2 specimen collection.Int J Infect Dis. 2021 Aug;109:195-198. doi: 10.1016/j.ijid.2021.06.060. Epub 2021 Jul 1. Int J Infect Dis. 2021. PMID: 34216732 Free PMC article.
-
Usability of an At-Home Anterior Nares SARS-CoV-2 RT-PCR Sample Collection Kit: Human Factors Feasibility Study.JMIR Hum Factors. 2021 Dec 14;8(4):e29234. doi: 10.2196/29234. JMIR Hum Factors. 2021. PMID: 34609947 Free PMC article.
-
3D-printed simulator for nasopharyngeal swab collection for COVID-19.Infect Dis Now. 2022 May;52(3):138-144. doi: 10.1016/j.idnow.2022.02.002. Epub 2022 Feb 8. Infect Dis Now. 2022. PMID: 35149235 Free PMC article.
References
-
- WHO Laboratory Testing for 2019 Novel Coronavirus (2019-nCoV) in Suspected Human Cases Interim Guidance. [(accessed on 17 January 2020)]; Available online: https://www.who.int/publications-detail/laboratory-testing-for-2019-nove....
-
- Dhiman N., Miller R.M., Finley J.L., Sztajnkrycer M.D., Nestler D.M., Boggust A.J., Jenkins S.M., Smith T.F., Wilson J.W., Cockerill F.R., III, et al. Effectiveness of patient-collected swabs for influenza testing. Mayo Clin. Proc. 2012;87:548–554. doi: 10.1016/j.mayocp.2012.02.011. - DOI - PMC - PubMed
-
- Elliot A.J., Bermingham A., Charlett A., Lackenby A., Ellis J., Sadler C., Sebastianpillai P., Powers C., Foord D., Povey E., et al. Self-Sampling for community respiratory illness: A new tool for national virological surveillance. Eurosurveillance. 2015;20:1–5. doi: 10.2807/1560-7917.ES2015.20.10.21058. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

