Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis
- PMID: 32916911
- PMCID: PMC7558351
- DOI: 10.3390/jdb8030018
Cranial Neural Crest Cells and Their Role in the Pathogenesis of Craniofacial Anomalies and Coronal Craniosynostosis
Abstract
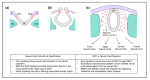
Craniofacial anomalies are among the most common of birth defects. The pathogenesis of craniofacial anomalies frequently involves defects in the migration, proliferation, and fate of neural crest cells destined for the craniofacial skeleton. Genetic mutations causing deficient cranial neural crest migration and proliferation can result in Treacher Collins syndrome, Pierre Robin sequence, and cleft palate. Defects in post-migratory neural crest cells can result in pre- or post-ossification defects in the developing craniofacial skeleton and craniosynostosis (premature fusion of cranial bones/cranial sutures). The coronal suture is the most frequently fused suture in craniosynostosis syndromes. It exists as a biological boundary between the neural crest-derived frontal bone and paraxial mesoderm-derived parietal bone. The objective of this review is to frame our current understanding of neural crest cells in craniofacial development, craniofacial anomalies, and the pathogenesis of coronal craniosynostosis. We will also discuss novel approaches for advancing our knowledge and developing prevention and/or treatment strategies for craniofacial tissue regeneration and craniosynostosis.
Keywords: craniofacial; craniofacial abnormalities; craniosynostoses; growth and development; neural crest; skull.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Morphological comparison of the craniofacial phenotypes of mouse models expressing the Apert FGFR2 S252W mutation in neural crest- or mesoderm-derived tissues.Bone. 2014 Jun;63:101-9. doi: 10.1016/j.bone.2014.03.003. Epub 2014 Mar 13. Bone. 2014. PMID: 24632501 Free PMC article.
-
Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis.Hum Mol Genet. 2006 Apr 15;15(8):1319-28. doi: 10.1093/hmg/ddl052. Epub 2006 Mar 15. Hum Mol Genet. 2006. PMID: 16540516
-
Face off against ROS: Tcof1/Treacle safeguards neuroepithelial cells and progenitor neural crest cells from oxidative stress during craniofacial development.Dev Growth Differ. 2016 Sep;58(7):577-85. doi: 10.1111/dgd.12305. Epub 2016 Aug 2. Dev Growth Differ. 2016. PMID: 27481486 Free PMC article. Review.
-
The Development of the Calvarial Bones and Sutures and the Pathophysiology of Craniosynostosis.Curr Top Dev Biol. 2015;115:131-56. doi: 10.1016/bs.ctdb.2015.07.004. Epub 2015 Oct 1. Curr Top Dev Biol. 2015. PMID: 26589924 Review.
-
Augmentation of BMP Signaling in Cranial Neural Crest Cells Leads to Premature Cranial Sutures Fusion through Endochondral Ossification in Mice.JBMR Plus. 2023 Feb 23;7(4):e10716. doi: 10.1002/jbm4.10716. eCollection 2023 Apr. JBMR Plus. 2023. PMID: 37065628 Free PMC article.
Cited by
-
Joint multi-ancestry and admixed GWAS reveals the complex genetics behind human cranial vault shape.Nat Commun. 2023 Nov 16;14(1):7436. doi: 10.1038/s41467-023-43237-8. Nat Commun. 2023. PMID: 37973980 Free PMC article. Review.
-
Single-cell atlas of early chick development reveals gradual segregation of neural crest lineage from the neural plate border during neurulation.Elife. 2022 Jan 28;11:e74464. doi: 10.7554/eLife.74464. Elife. 2022. PMID: 35088714 Free PMC article.
-
Cranial Suture Mesenchymal Stem Cells: Insights and Advances.Biomolecules. 2021 Jul 31;11(8):1129. doi: 10.3390/biom11081129. Biomolecules. 2021. PMID: 34439795 Free PMC article. Review.
-
Essential function and targets of BMP signaling during midbrain neural crest delamination.Dev Biol. 2021 Sep;477:251-261. doi: 10.1016/j.ydbio.2021.06.003. Epub 2021 Jun 6. Dev Biol. 2021. PMID: 34102166 Free PMC article.
-
Cranium growth, patterning and homeostasis.Development. 2022 Nov 15;149(22):dev201017. doi: 10.1242/dev.201017. Epub 2022 Nov 21. Development. 2022. PMID: 36408946 Free PMC article.
References
-
- Lin Y., Lewallen E.A., Camilleri E.T., Bonin C.A., Jones D.L., Dudakovic A., Galeano-Garces C., Wang W., Karperien M.J., Larson A.N., et al. RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J. Orthop. Res. 2016;34:1950–1959. doi: 10.1002/jor.23209. - DOI - PMC - PubMed
-
- Bianchi F., Calzolari E., Ciulli L., Cordier S., Gualandi F., Pierini A., Mossey P.A. Environment and genetics in the etiology of cleft lip and cleft palate with reference to the role of folic acid. Epidemiol. Prev. 2000;24:21–27. - PubMed
-
- Dixon J., Jones N.C., Sandell L.L., Jayasinghe S.M., Crane J., Rey J.P., Dixon M.J., Trainor P.A. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc. Natl. Acad. Sci. USA. 2006;103:13403–13408. doi: 10.1073/pnas.0603730103. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

